Fastened Tight for Better Contrast
Sensitivity of magnetic resonance imaging (MRI) contrast agents raised through multiple bonds to a protein
magnetic resonance imaging (MRI) has developed into an important, widely used diagnostic technique. Complexes of the rare-earth metal gadolinium are used as contrast agents. American researchers have now developed a way to increase the sensitivity of these contrast agents: they fasten a chain of several gadolinium complexes to a protein from the tissue that is to be examined.
MRI is based on the intrinsic angular momentum, or spin, of hydrogen atom nuclei. In order to make individual organs visible, the contrast agent must mark them specifically. For example, the contrast agent Vasovist (MS-325) targets blood vessels, making them visible for the study of vascular diseases. Vasovist is a gadolinium complex with a kind of molecular anchor that binds specifically to albumin, a protein present in blood serum. When bound to this large protein, rotation of the gadolinium complex around its own axis is dramatically slowed and yields a stronger signal. A fast rotating contrast agent is less effective in "shaking up" the proton spins, so binding to the target protein improves the effectiveness of the contrast agent, essentially "switching" it on.
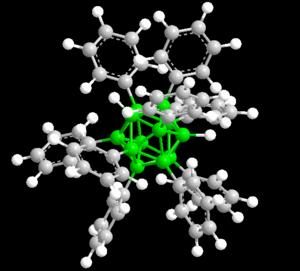
Peter Caravan and a team of researchers at EPIX Pharmaceuticals and New York Medical College wanted to increase the sensitivity of MRI for potentially broader applications. Would it be possible to improve the contrast agent by increasing the number of gadolinium complexes? The scientists fastened several gadolinium complexes into a chain with an anchor for albumin at the end, as before. The disadvantage to this approach is that the individual gadolinium complexes within the chain become mobile and able to turn again, putting the desired improvement in contrast out of reach. However, the researchers found a clever way around this. They simply bound a second anchor to the other end of the chain so that the chain binds to the albumin in two places. The chain is thus fastened tightly. The severely reduced mobility of the complexes in the chain is indeed reflected in the significantly increased contrast.
Original publication: P. Caravan; "Multilocus Binding Increases the Relaxivity of Protein-Bound MRI Contrast Agents"; Angewandte Chemie International Edition, doi: 10.1002/anie.200502245
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Whey_protein
European_Medical_Students'_Association