Gilead Delivers Termination Notice to Roche for Tamiflu Development and Licensing Agreement
Gilead Sciences, Inc. announced that it has delivered a notice of termination to F. Hoffmann-La Roche Ltd (Roche) for material breach of the parties' 1996 Development and License Agreement for Tamiflu(R) (oseltamivir phosphate), an antiviral pill for the treatment and prevention of influenza. Through this action, Gilead is seeking to terminate the 1996 agreement, which would result in the rights to Tamiflu held by Roche reverting to Gilead.
Tamiflu is the only antiviral pill that has demonstrated activity against the most common strains of the virus and is approved for both the treatment and prevention of influenza infection. Ensuring that Tamiflu is made as widely available as possible is necessary for the protection of public health. Gilead intends to provide physicians, public health officials and consumers with greater access to and information about Tamiflu.
"Despite our repeated communication of concerns over the last several years, Roche has not adequately demonstrated the requisite commitment to Tamiflu since its launch in the United States nearly six years ago, nor has it allocated the necessary resources to realize the potential of the product as a treatment and preventive for influenza," said John C. Martin, PhD, President and Chief Executive Officer, Gilead Sciences. "Gilead is taking this action in the interest of our shareholders and, importantly, because it is essential for public health that healthcare professionals and consumers have improved access to information about Tamiflu, as well as to the product itself."
Gilead's notice of termination describes material breaches of obligations by Roche under the 1996 Agreement in the following areas: (1) Roche's failure to use best efforts to commercialize Tamiflu by adequately and sustainably promoting and marketing the product in all significant markets, including the failure to launch in a number of markets where the product has been approved; (2) Roche's failure to use best efforts to commercialize Tamiflu as evidenced by past problems with the manufacturing process that led to shortages in product supply; and (3) Roche's failure to properly calculate and pay the royalties fairly owed to Gilead.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
MorphoSys Signs Manufacturing Agreement with Boehringer Ingelheim
Orange_G

World-first Panama disease-resistant Cavendish bananas
List_of_subjects_in_Gray's_Anatomy:_IX._Neurology
Richard_Evans_Schultes
Grimm's_hydride_displacement_law
Category:Smallpox
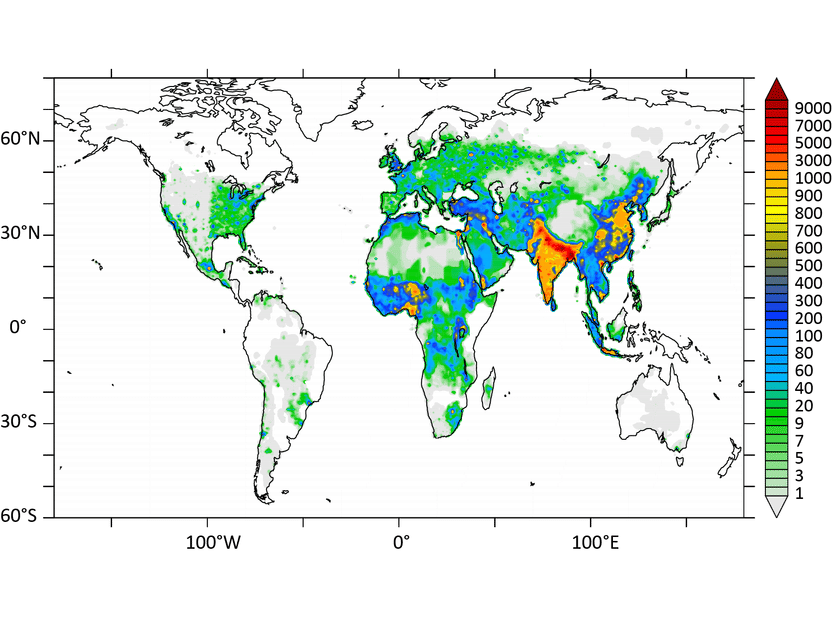
More deaths due to air pollution - Air pollution could claim 6.6 million lives by 2050
Riemser Arzneimittel AG Sells Veterinary Business to Ecuphar N.V.
Primary endpoint reached in phase II clinical trial of Active Biotech's substance for oral treatment of multiple sclerosis
Genetic Study to Understand Linkage Between Lead Exposure and Children's Intellectual Development in India - BioServe Partners with Harvard and University of Michigan on Landmark Public Health Study























































