New Generation BSE test approved by CFIA
Prionics AG announced that the Canadian food Inspection Agency (CFIA) has granted approval of the Prionics(R)-Check PrioSTRIP, the latest generation of testing technology for Bovine Spongiform Encephalopathy (BSE).
A breakthrough in BSE testing, the Prionics(R)-Check PrioSTRIP combines speed with reliability. The test, which represents an innovative mass- screening application of the proven lateral flow technology, is simple, easy and fast providing results in about 100 minutes. It received EU approval in February 2005 and has been successfully introduced in European laboratories.
The Prionics(R)-Check PrioSTRIP represents a considerable improvement over existing BSE testing platforms, which typically deliver results between 4 to 5 1/2 hours. It allows high throughput processing with minimal requirements for specialized equipment. Also, the use of the Prionics(R)-Check PrioSTRIP requires only minimal training of laboratory personnel while delivering the highest standards of sensitivity and specificity.
Other news from the department research and development
These products might interest you

Systec H-Series by Systec
Safe, reproducible and validatable sterilization of liquids, solids and waste
Autoclaves with 65-1580 liters usable space, flexibly expandable for various applications

Whatman™ folded filter papers by Cytiva
Whatman folded filter papers
Convenient folded formats speed up your sample preparation

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Propiconazole
ClinPhone Introduces New State-of-the-Art Data Center Following Recent Influx of New Business
11Beta_Hydroxysteroid_dehydrogenase
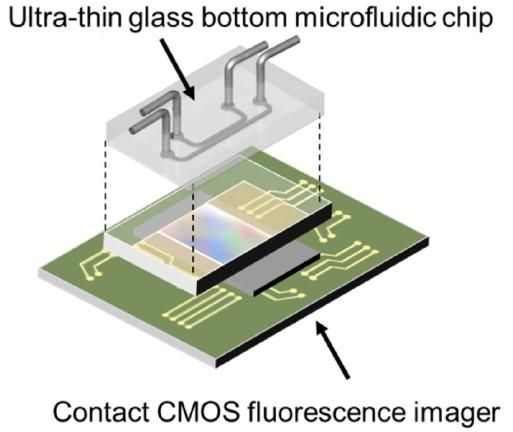
Fluorescence microscopy on a chip -- no lenses required
Syntaxin appoints Executive Chairman
Biogen Idec and Sobi announce positive top-line results from phase 3 study in hemophilia B
Carl Zeiss Meditec AG wins patent infringement action on trifocal intraocular lens






















































