ALTANA Pharma and Atugen Renew Target Validation and License Agreement
Atugen AG announced that it has renewed its non-exclusive target validation and License Agreement with ALTANA Pharma AG for another year. The agreement gives Altana the right to have molecular targets validated by Atugen's discovery team, utilizing a number of proprietary knockdown tools and transfection technologies. Under the agreement, Altana also receives a license to use Atugen's gene silencing and oligonucleotide delivery technologies. The license was first granted in August 2001 and Altana has paid Atugen several research milestones.
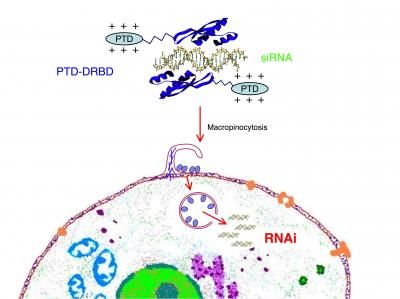
Atugen uses a unique target validation approach where both siRNA molecules and a third generation antisense technology (GeneBloc®) are employed in parallel. This approach, called CrossValidation, is designed to exclude artifacts and nonspecific effects that could arise if either technology were applied alone. The GeneBloc® technology is, in contrast to other antisense technologies, extremely potent and devoid of toxicity. Furthermore, Atugen possesses a set of proprietary liposomal transfection reagents that is custom-tailored to individual cell lines and minimizes toxicity while obtaining high transfection rates.
Atugen's gene silencing technologies are employed in in vitro and in vivo target validation and the Company has a program to develop siRNA therapeutics.
Most read news
Topics
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.