SGX and CFFT announced a discovery that is likely to open up new ways in treating cystic fibrosis
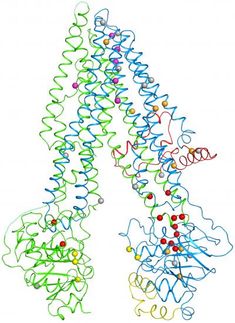
Structural GenomiX, Inc. (SGX ) and cystic fibrosis Foundation Therapeutics, Inc. (CFFT) announced a discovery that may open up new ways to treat cystic fibrosis (CF). The findings, published in the November 4 advance online publication of the Journal of Biological chemistry, were made through the investigation of the three-dimensional crystal structure of a region of the human Cystic Fibrosis Transmembrane conductance Regulator (CFTR) protein implicated in the onset of cystic fibrosis, known as nucleotide binding domain 1 (NBD1).
The analysis documented that the most common inherited mutation causing CF creates a small, but critical change in the surface of NBD1 that may alter the way this domain of the CFTR protein interacts with other domains of CFTR. It also could influence the way the CFTR protein interacts with other important regulatory proteins in the cells of people living with CF.
Ninety percent of people with CF carry this common type of disease-causing mutation, identified as Delta F508, in the NBD1 region of the CFTR protein. This work by SGX scientists represents the first time that the NBD1 of human CFTR has been solved. This new information is expected to guide additional research needed to discover an effective treatment for CF. Researchers think that while it may have been very difficult to develop drugs that would address a large conformational change to CFTR, it may be more feasible to develop drugs that target this small change.
"SGX's first-ever determination of the crystal structure of the disease-causing mutant form of NBD1 is an important finding and a strong foundation for our future drug discovery and development initiatives," said Robert J. Beall, Ph.D., president and chief executive officer of the CF Foundation and CFFT. "While many of our drug development initiatives still are viable potential therapies, this discovery will help us narrow our search for drug candidates that address this specific change."
"The achievement of this medically-important discovery made under our collaboration with CFFT clearly demonstrates the power and versatility of the SGX technology platform and its ability to impact drug discovery for life-threatening diseases such as CF," said Stephen K. Burley, M.D., D.Phil., chief scientific officer of SGX. "With drug targets as challenging as the CFTR protein, experimentally determined structures will be invaluable as they allow researchers to see in three-dimensions precisely how small molecules bind to the target protein and help correct functional defects arising from disease-causing mutations."
SGX has been collaborating with CFFT since early 2001 to apply its state-of-the-art technology platform for elucidation of CFTR's three-dimensional structure. The achievement of this milestone resulted in a payment from CFFT to SGX.
The NBD1 structural information produced by SGX has been deposited in its entirety in the Protein Data Bank which maintains a comprehensive, publicly accessible archive of three-dimensional structures of biological macromolecules.
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Prosensa receives £7.5m milestone payment as part of its program with GlaxoSmithKline in Duchenne Muscular Dystrophy
How our nerves regulate insulin secretion
Reserpine
Titer
Hypohidrotic_ectodermal_dysplasia
Macrovipera_mauritanica
Unconscious learning uses old parts of the brain
Dextro-Transposition_of_the_great_arteries

LabRAM Soleil | Raman spectrometers | Horiba