GE Healthcare Announces Licensing Agreements with Bristol-Myers Squibb and Regeneron Pharmaceuticals for Green Fluorescent Prote
Access to GFP technology aids drug discovery and development
Advertisement
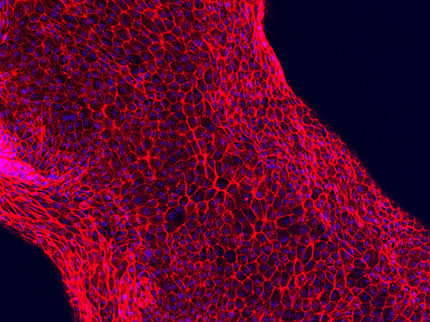
Chalfont St. Giles, UK: GE Healthcare, a combination of the former Amersham and GE Medical Systems, today announced that Bristol-Myers Squibb and Regeneron Pharmaceuticals have each signed licensing agreements for rights to Aequorea Victoria Green Fluorescent Protein (AvGFP), the most versatile, widely used and well validated fluorescent protein on the market. GE Healthcare has the exclusive rights to offer comprehensive licensing for the intellectual property necessary to make the best use of this GFP technology.
The licensing agreement signed by Bristol-Myers Squibb entitles it to use GFP to carry out primary screening, secondary screening, profiling, research and lead optimisation activities for the development of human therapeutics and human prophylactics, and in the construction of transgenic animal lines. Bristol-Myers Squibb has also secured rights to operate under BioImage's Redistribution(tm) patent portfolio for monitoring cellular signalling pathways.
Under its license agreement, Regeneron will use GFP in the discovery and development of human therapeutics. It also gives Regeneron the rights to commercialise cell lines it has developed to produce therapeutic proteins and to develop further cell lines.
"Bristol-Myers Squibb and Regeneron join a growing number of world-leading pharmaceutical and biotechnology companies that are using the power of GFP technology," said Mike Evans, vice president discovery systems marketing and strategy at GE Healthcare. "By using GFP to label target proteins, researchers can track proteins in living cells and screen for compounds that affect specific cellular signaling pathways. This improves the biological relevance of drug screening and helps increase the speed and accuracy of drug discovery and development."
GFP is compatible with most fluorescent micro and macro imagers as well as plate readers. When used in conjunction with the IN Cell Analyzer high-throughput sub-cellular imaging system from GE Healthcare, GFP technology allows scientists to look inside live cells, study how proteins move about and function, and evaluate how drug candidates affect cellular processes.