QIAGEN and ARTUS Announce Supply Agreement to Provide QIAamp Sample Preparation Kits for Use with Artus' Diagnostic Products
Advertisement
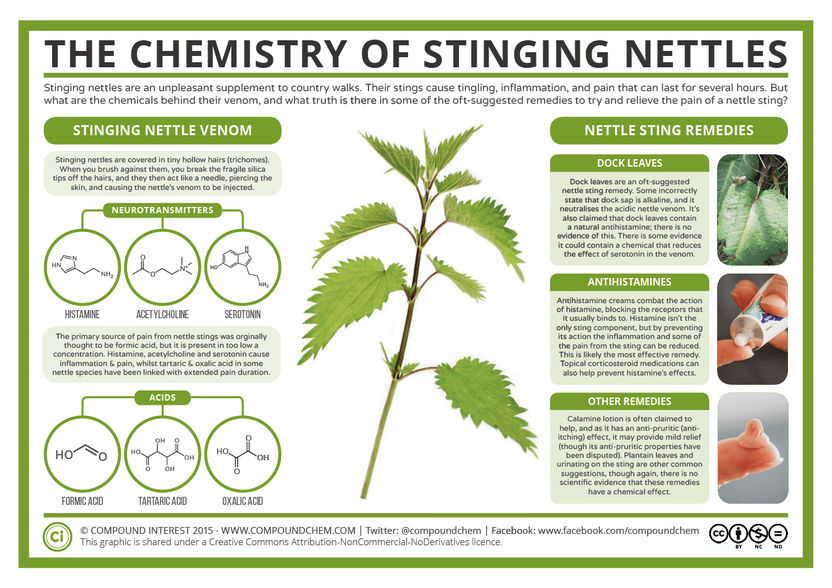
QIAGEN GmbH and artus GmbH announced that they have entered into a manufacturing and supply agreement. Pursuant to this agreement, artus will integrate QIAGEN's nucleic acid sample preparation products in its diagnostic systems for use in the detection of a variety of infectious diseases such as SARS, herpes simplex virus -1/-2, EBV, West Nile Virus, malaria, Salmonella. These diagnostic solutions employ artus' RealArt® amplification technology which is based on the polymerase chain reaction (PCR) technology to which artus holds a non-exclusive license from Roche.
The customized solutions will incorporate specific versions of QIAGEN's sample preparation modules and technologies, including QIAGEN's well established QIAamp technology and will be distributed by artus and its distributors in combination with the real-time PCR-based diagnostic solutions under the brand PureArt.
QIAGEN's proprietary nucleic acid sample preparation and handling technologies provide high reliability and efficiency in both, DNA and RNA separation and purification in molecular diagnostics. Following nucleic acid sample extraction, processed samples will be transferred onto artus' RealArt system for amplification and detection.
"We are very pleased that artus has selected QIAGEN nucleic acid purification kits as a front-end preparation solution to their real-time amplification and detection system," said Dr. Frank Krieg-Schneider, Director Strategic Alliances at QIAGEN. "Artus is a visionary and technology-leading molecular diagnostics company and its RealArt® solutions are considered some of the most sensitive and efficient diagnostic solutions. Efficient extraction and purification of target nucleic acids is key to the sensitivity and reliability of nucleic acid testing. Methods such as PCR possess significant and technological challenges in clinical settings. QIAGEN's technology and market leadership in nucleic acid purification in molecular diagnostics is adding substantial value to the development of standardized and highly efficient solutions in this emerging and rapidly growing market."
"We are very happy about the supply agreement we have signed with QIAGEN", said Dr. Ulrich Spengler, managing director, artus GmbH. "QIAGEN's expertise in nucleic acid extraction and purification reflected in their sample preparation modules and technologies addresses the requirements in reliability and efficiency of RNA and DNA preparation in molecular diagnostics and will add significant value to our RealArt PCR assays."