New study identifies targets to lessen the effects of alcoholic liver disease
In 2015, nearly half of 80,000 deaths due to liver diseases in the United States were related to alcoholic liver disease (ALD), according to the National Institute on alcohol Abuse and alcoholism. Chronic alcohol consumption causes abnormal fat accumulation in liver cells (steatosis) and liver fibrosis, which can lead to hepatitis, cirrhosis, and sometimes liver cancer. A new study in The American Journal of Pathology offers insights into the cellular aging that may trigger excessive fibrosis formation in the liver as well as possible means to inhibit these changes, which may lead to new therapeutic approaches for patients with ALD.
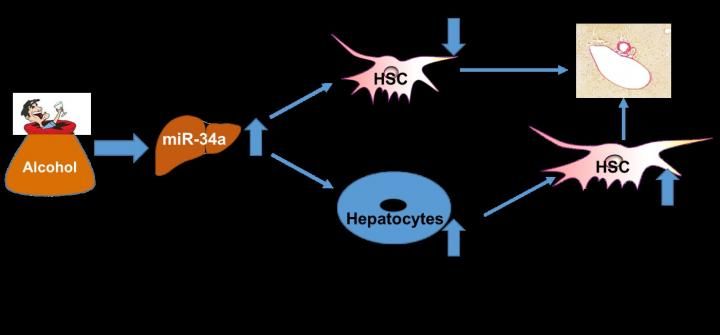
Up-regulation of miR-34a contributes to liver fibrosis during alcoholic liver disease by increasing senescence of hepatocytes and decreasing senescence of hepatic stellate cells (HSC).
Ying Wan
"We believe that senescent cells contribute to age-related tissue degeneration during chronic liver injuries," explained co-author Fanyin Meng, MD, PhD, Associate Professor of Internal Medicine at Baylor Scott & White Digestive Disease Research Center (BSWDDRC), Texas A&M College of Medicine and Central Texas Veterans Health Care System, Temple, TX. "Cellular senescence refers to a state of irreversible cell-cycle arrest combined with the secretion of proinflammatory cytokines and hepatocellular dysfunction. Our study demonstrates that the drivers of aging are critical mediators of ALDs."
Investigators studied liver tissue from patients with steatohepatitis, who were heavy alcohol drinkers, and from ethanol-fed mice to identify biochemical markers of cellular senescence. Their findings indicate that up-regulation of microRNA-34a (miR-34a) during alcohol consumption contributes to the development of liver fibrosis during alcoholic liver injury. The fibrosis-producing effects of miR-34a are related to different aging signaling in two different liver cell types. Particularly, in hepatocytes, the primary liver cells that make up 70% to 85% of the liver's mass and perform the basic functions of the liver, senescence is increased. On the contrary, senescence is decreased in activated hepatic stellate cells (HSCs), the supportive cells which, when triggered by alcohol or other liver insults, begin to produce excessive fibrotic material. Their research also shows that inhibition of hepatic miR-34a expression reduces liver injury and liver fibrosis in ALD.
"Understanding the mechanisms underlying HSC activation and regression has become an increased area of interest, and our findings help to advance understanding of the complex nature of this phenomenon," noted Dr. Meng.
On a broader scale, the study identifies a novel pathway by which HSC activation and regression are regulated, which could potentially be applied to other aging-associated fibrotic liver diseases. "Targeting the drivers of aging and senescent cells may be a novel therapeutic strategy to reduce hepatic steatosis and liver fibrosis in ALD patients," commented co-author Gianfranco Alpini, PhD, Distinguished Professor in the Department of Medical Physiology at Texas A&M College of Medicine, Senior Research Scientist at Central Texas Veterans Health Care System, and Director of the BSW DDRC.
Further, co-author Heather Francis, PhD, Associate Professor of Medical Physiology at Texas A&M College of Medicine and member of the BSWDDRC, stated, "It is imperative to identify regulatory targets for potential treatment of ALDs, especially for populations that are greatly impacted by this disease. Moreover, Shannon Glaser, PhD, Associate Professor of Medical Physiology at Texas A&M College of Medicine and member of the BSWDDRC added, "Targeting the miR-34a may also be key for managing liver fibrosis in other cholangiopathies such as primary sclerosing cholangitis as well as primary biliary cholangitis. Our study opens the window for the possibility of linking age-related genes as therapeutics for the future."
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Transgene to Collaborate in a Novel Melanoma Treatment Under a Cooperative Research and Development Agreement

ONE microscopy: from molecule to 3D structure with conventional microscopy - The simple and cost-effective method developed using a few simple but effective tricks
Evotec receives milestone payment for start of second Phase II trial in its multi-target alliance with Bayer