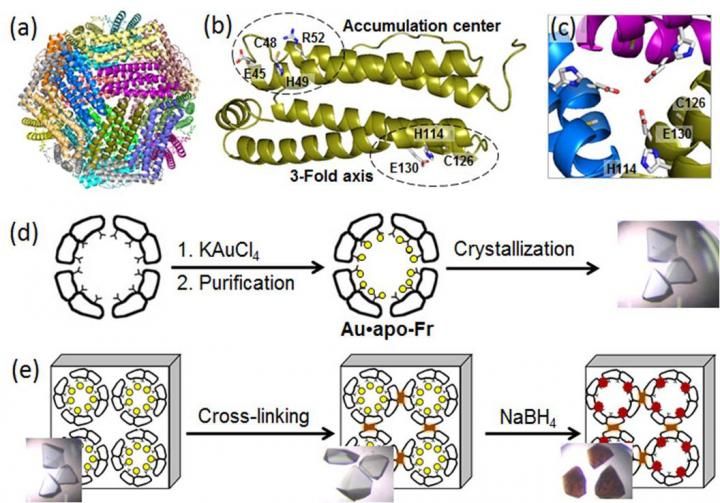
Recipharm equips Lisbon facility for US and European serialisation
Recipharm, a leading contract development and manufacturing organisation (CDMO) has equipped its sixth facility with serialisation capabilities ahead of the US and European regulation enforcement deadlines.
The facility in Lisbon is the latest of Recipharm’s facilities to be prepared to start supplying serialised products to the US and Europe, following a EUR 40 million investment into its company-wide implementation programme in 2016.
Recipharm has already delivered over 1.3 million serialised and aggregated packs to markets such as, China, South Korea, Saudi Arabia and Turkey where serialisation regulations are currently in place. The Lisbon site is the sixth to become serialisation-ready for the US and European markets and adds an additional four packaging lines to the company’s current serialisation capabilities. This will be followed by a further seven lines at the facility by Q2 in 2018.
Staffan Widengren, Director of Corporate Projects at Recipharm and head of the global steering committee for Recipharm’s serialisation project said: “Recipharm recognised the complexity of implementing serialisation at a very early stage and so we’ve been preparing for the new regulations in the US and Europe for a long time now. The Lisbon facility brings us to over a third of the way through our implementation project and is an important milestone in our journey.”
“We introduced our serialisation programme to ensure a consistent roll-out of our standard solution, without the implementation process having a significant impact on production activities. It’s important our solution works at a local level, as well as company-wide, and the central team that heads up the programme is tasked with making this happen.”
“Effective serialisation capabilities can eventually help companies to improve their overall equipment effectiveness (OEE) and streamline their operations. The programme also helps us to ensure that serialisation data integrates with our enterprise resource planning (ERP) activity and our manufacturing execution systems (MES) so that we can achieve wider business benefits beyond compliance.”
The central team is responsible for identifying, ordering, installing and qualifying serialisation systems in line with customer requirements. The next site to be equipped will be the company’s facility in Brescia, which will supply serialised product to the US.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Palmar_plantar_erythrodysesthesia
Alagille_syndrome
Tony_Coelho
Stem-cell-growing surface enables bone repair
CHARGE_syndrome
Stretched, ordered DNA molecules could bring insights into disease
Study uncovers new hurdle for developing immunotherapies
How a molecular Superman protects the genome from damage - Scientists find a new role for RNAi protein Dicer in preventing collisions during DNA replication
Networks of Gene Activity Control Organ Development
AIDS_advocacy
Evotec completes acquisition of Rigenerand