A step towards a new drug to treat fungal infections that kill 1.6 million people annually
Advertisement
A team from Sydney's Westmead Institute for Medical Research is a step closer to developing a drug to treat life-threatening fungal infections that cause more than 1.6 million deaths annually.
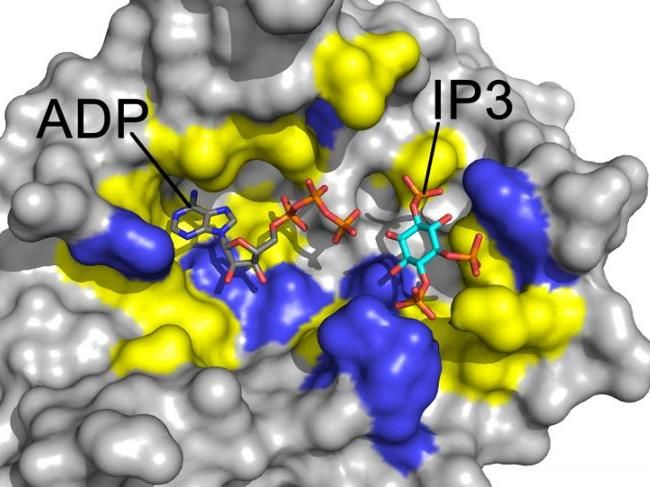
Yellow highlights the differences in fungal Arg1 that can be exploited for drug development.
Professor Joel Mackay, School of Life and Environmental Sciences at the University of Sydney
Death rates due to fungal infections are similar to those of tuberculosis and greater than those due to malaria.
The team discovered that Arg1, a protein produced by all fungi, enables the Cryptococcus neoformans fungus to establish potentially lethal infections in patients with weakened immune systems, such as those with HIV/AIDS or leukaemia.
Lead researcher, Associate Professor Julianne Djordjevic, said she is optimistic this discovery will provide a new avenue for the development of safer urgently needed antifungal drugs.
"Finding new treatments to kill fungi is a major health priority. Novel antifungal drugs are desperately needed to reduce the high global morbidity, mortality and cost associated with treating invasive fungal infections.
"Because fungi are so similar to human cells, developing new drugs that kill fungi - but are non-toxic to humans - is a challenge. Furthermore not all antifungal drugs kill all types of fungi and drug-resistance is an emerging problem.
"By investigating how fungi cause disease, we have identified a new drug target with the view to design new therapies to combat these serious infections," she said.
Cryptococcus neoformans is the world's most common cause of fungal meningitis.
The primary site of infection is the lung. After inhalation from the environment, the infection spreads from the lung to the brain via the bloodstream.
Immunosuppressed patients typically present with meningitis, which is fatal without treatment.
Associate Professor Djordjevic and her team identified the Arg1 protein as part of their investigation of the mechanisms that fungi exploit to cause life-threatening infections in the body.
"Our laboratory has established reliable methods for studying Cryptococcus neoformans at the molecular level.
"Using gene deletion analysis and animal infection models, we identified that the Arg1 metabolic pathway allows Cryptococcus to establish a lung infection and to spread to brain, resulting in meningitis.
"We are now looking at Arg1 as a selective target for antifungal drug development. By targeting Arg1, we hope to block the Arg1 metabolic pathway and thereby prevent Cryptococcus from establishing a lung infection and spreading to the brain," Associate Professor Djordjevic explained.
Associate Professor Djordjevic said that even with appropriate treatment, high mortalities are reported highlighting the need to develop more effective antifungal therapies.
"Fungal diseases are a growing global health problem and a leading cause of blindness, respiratory disease, meningitis and death.
"Treatments with fewer side effects will significantly increase the chances of recovery for a patient, potentially saving thousands of lives every year," Associate Professor Djordjevic concluded.
Original publication
Cecilia Li, Sophie Lev, Desmarini Desmarini, Keren Kaufman-Francis, Adolfo Saiardi, Ana P.G Silva, Joel P. Mackay, Philip E. Thompson, Tania C. Sorrell & Julianne T. Djordjevic; "IP3-4 kinase Arg1 regulates cell wall homeostasis and surface architecture to promote clearance of Cryptococcus neoformans infection in a mouse model"; Virulence; 2017