Adjustment of drug dose for rheumatoid arthritis by diagnostic method
Determination of the therapeutic window of adalimumab
Collaboration between the Immanuel Klinikum Bernau Herzzentrum Brandenburg, the Rheumazentrum Nord-Brandenburg and BioTeZ demonstrates new ways of treating rheumatoid arthritis.
Graphical summary of the results
BioTeZ
Through the introduction of therapeutic antibodies, the treatment results for rheumatoid arthritis (RA) have been significantly improved over the past decade. However, cases of inadequate effectiveness remain, as well as side effects due to the associated strong immunosuppression, especially with high activity levels. One possibility for therapy optimization is the targeted, individual dose adjustment of the active ingredients for each patient. At the moment, all RA patients treated with the TNF-alpha inhibitor adulimumab received a pre-approved standard dose of 40 mg every 14 days without regard for existing differences in body weight, sex, health status, metabolism and age. As a result, some patients receive too high drug dose and other patients have too low drug levels.
Using an ELISA-test from BioTeZ, it is now possible simultaneously quantify the concentrations of the therapeutic antibody adalimumab and the target antigen TNF alpha in the patient's serum during therapy.
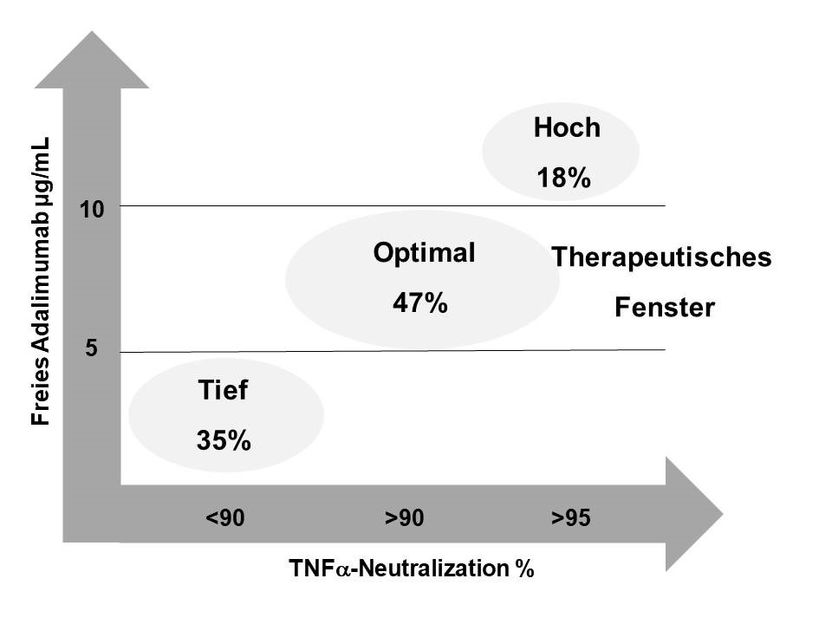
A total 47% of the patients showed drug levels in the target area, leading to an effective neutralization of TNF-alpha. 35% of the patients showed low drug doses resulting in reduced TNF alpha neutralization rates under standard therapy. 18% of patients had increased drug levels and high neutralization rates. A further dose increase for these patients is neither useful nor recommended.
"If the effect of the drug is insufficient and the drug level is low, a dose increase of the adalimumab by a weekly injection can lead to a significantly better therapeutic response (Zänker et al., 2017). This is in line with our everyday experience in the clinics and has now been scientifically documented in this study", says Dr. Michael Zänker, rheumatologist and chief physician for internal medicine at Immanuel Klinikum Bernau Herzzentrum Brandenburg.
In the case of very high drug levels, as in 18% of the patients and if the therapeutic goal is achieved, there is the possibility of de-escalating the therapy, by stretching the interval of the injections by several days. Thus the amount of active substance in the patient can be reduced to the recommended values, while avoiding possible side effects as well as reducing treatment costs.
"The results contribute to the individual treatment of patients with rheumatoid arthritis and are an important add on to the therapy prediction of anti-TNF-alpha monotherapy with adalimumab" so, Dr. Janko Brand, scientific director of the study at BioTeZ.
The study was funded by the Rheumazentrum Nordbrandenburg e.V. The results of more elaborated studies are currently being evaluated and new customer projects for further biologics are being developed.
Original publication
Zänker, Michael and Becher, Gunther and Arbach, Olga and Maurer, Marcus and Stuhlmüller, Bruno and Schäfer, Astrid and Strohner, Pavel and Brand, Janko; "Improved adalimumab dose decision with comprehensive diagnostics data"; Clinical and experimental rheumatology; 2017
Most read news
Original publication
Zänker, Michael and Becher, Gunther and Arbach, Olga and Maurer, Marcus and Stuhlmüller, Bruno and Schäfer, Astrid and Strohner, Pavel and Brand, Janko; "Improved adalimumab dose decision with comprehensive diagnostics data"; Clinical and experimental rheumatology; 2017
Topics
Organizations
Other news from the department science
These products might interest you
Pharmaceutical Substances by Thieme Verlag
Look up Industrial Syntheses of 2,600 APIs
Your tool for Syntheses, Patents and Applications – Pharmaceutical Substances

KNAUER IJM NanoScaler by KNAUER
Efficient formulation of lipid nanoparticles for RNA-based therapies
Optimise drug encapsulation from 1 ml to hundreds of millilitres with minimal drug input

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Ovarian_cancer
Transporter of thyroid hormones crucial for embryonal brain development
List_of_notifiable_diseases
Scientists find key to regenerating blood vessels
How ethane-consuming archaea pick up their favorite dish - Scientists sucessfully decoded the structure of the enzyme responsible for ethane fixation

Plastic pollution harms bees: risks for global food security - A new review study is the first to systematically show the harmful effects of nano- and microplastics on bees and other beneficial insects
Williams_syndrome
List_of_subjects_in_Gray's_Anatomy:_V._Angiology
QIAGEN and Institute for Animal Health Enter Partnership in Bluetongue Testing