Fast, convenient & standardized: New lab innovation for automated tissue engineering & drug testing
MBM ScienceBridge GmbH successfully negotiated a license agreement between University Medical Center Göttingen (UMG) and the biotech company tissue Systems Holding GmbH about commercial use of a multi-well tissue plate for automated and reliable tissue engineering & drug testing. This lab innovation is based on previous scientific work from the group of Prof. Dr. Wolfram-Hubertus Zimmermann, Director of the Institute for Pharmacology and Toxicology at the UMG. Under the terms of the non-exclusive license agreement UMG will receive an upfront payment and, besides royalties, annual minimum license fees.
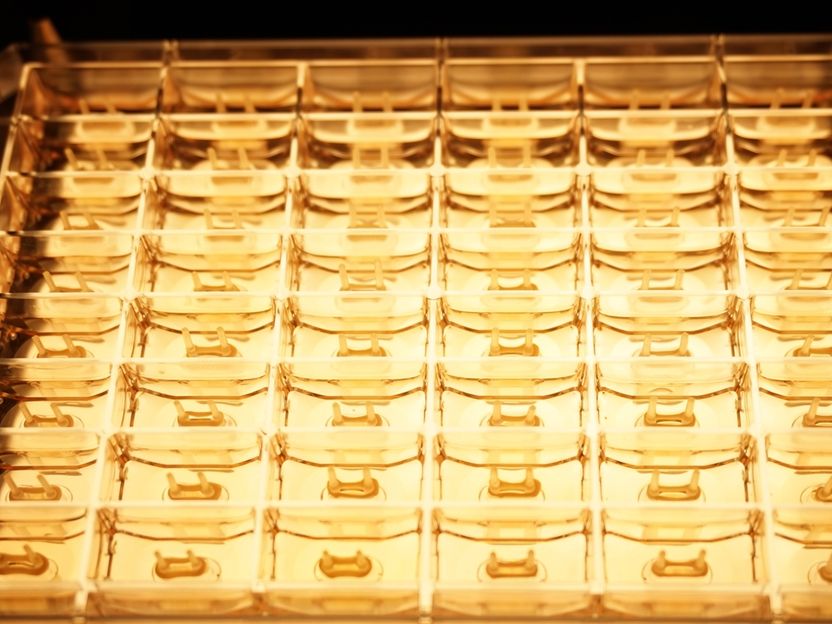
Multi-well tissue plate (48-well format) for automated cardiotoxic or drug screening.
T. Meyer
Unwanted side effects affecting the heart are among the most common causes for the high attrition rate in drug development. Furthermore, cardiovascular diseases are the major cause of death in Germany, accounting for almost 40% of the annual mortalities. Hence, preclinical testing for both cardiac toxicity as well as drug efficacy is an important precondition for successful drug development.
Currently, these tests are primarily performed in cell cultures or animal models. Because these models do not accurately reflect human physiology, there is a concern of limited predictive value as to clinical relevance of the obtained knowledge. Instead, tissue-engineered and lab-grown human tissues can much better reflect physiological conditions. The newly developed multi-well tissue plate at UMG (currently at a 48-well format) allows for a standardized generation of human heart muscle under defined conditions from stem cells in a simple one-step procedure. The culture format was designed to be ready for automation and analytic monitoring of tissue function without manual handling - enabling effective high-throughput drug testing. The platform can be easily adapted for use with other tissue formats.
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.