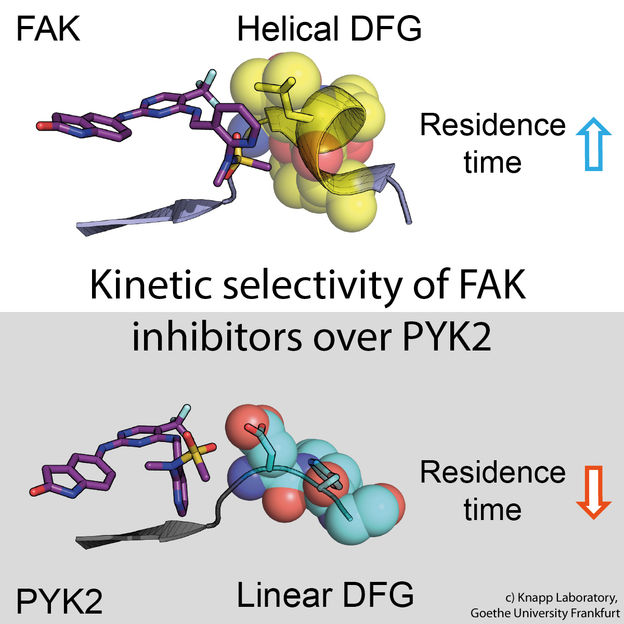
Recipharm and LIDDS establish industrial manufacturing capabilities
Manufacturing line for prostate cancer product set up
Recipharm and LIDDS have together set up a manufacturing line for LIDDS’ novel prostate cancer drug, Liproca®Depot, at Recipharm in Solna, Sweden.
The manufacturing line is dedicated to the first product based on LIDDS’ innovative NanoZolid®technology. Clinical trial material has already been produced and the facility is ready for future commercial manufacturing of Liproca Depot or other pharmaceutical formulations based on the NanoZolid technology. The manufacturing line has been adapted for GMP production and is industrialised according to a unique process invented by LIDDS, involving the installation of novel equipment that is new to the pharmaceutical industry.
”We are proud of having reached this important milestone in the development of a new product allowing improved therapy for a serious disease. This has been achieved by the hard work and close collaboration of experts at both LIDDS and Recipharm. LIDDS is a very innovative company and it has been extremely rewarding to work with the team”, says Torkel Gren, General Manager of Recipharm’s facility in Solna.
”With the successful manufacturing performed at Recipharm, LIDDS can offer license agreements for Liproca Depot with the advantage of having validated production in place, saving both time and money for pharma companies. In addition, we now have an established manufacturing facility ready for other NanoZolid projects”, says Monica Wallter CEO of LIDDS.
Most read news
Organizations
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

When and why does type 1 diabetes manifest in children? - New findings on the development of the autoimmune disease in children
Amylin Announces Positive Results from Dose-Ranging Clinical Study of Pramlintide/Metreleptin Combination Treatment for Obesity