Killing cancer in the heat of the moment
Advertisement
Mineko Kengaku, Tatsuya Murakami, and their colleagues from Kyoto University's Institute for Integrated Cell-Material Sciences (iCeMS) have developed a new method that modifies the surface of nanorods, making them more efficient in transporting cancer-killing genes into cells.
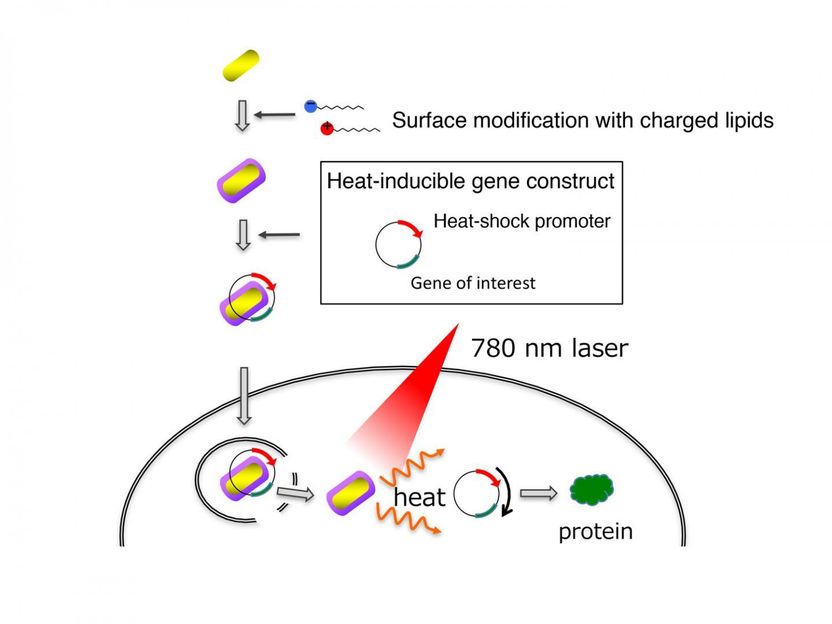
This is the delivery and activation of genes by gold nanorods. Gold nanorods coated with charged lipids efficiently bind to DNA and penetrate cells. The team designed an artificial gene that is turned on by heat generated by the gold nanorods upon exposure to near infrared light illumination.
Kyoto University iCeMS
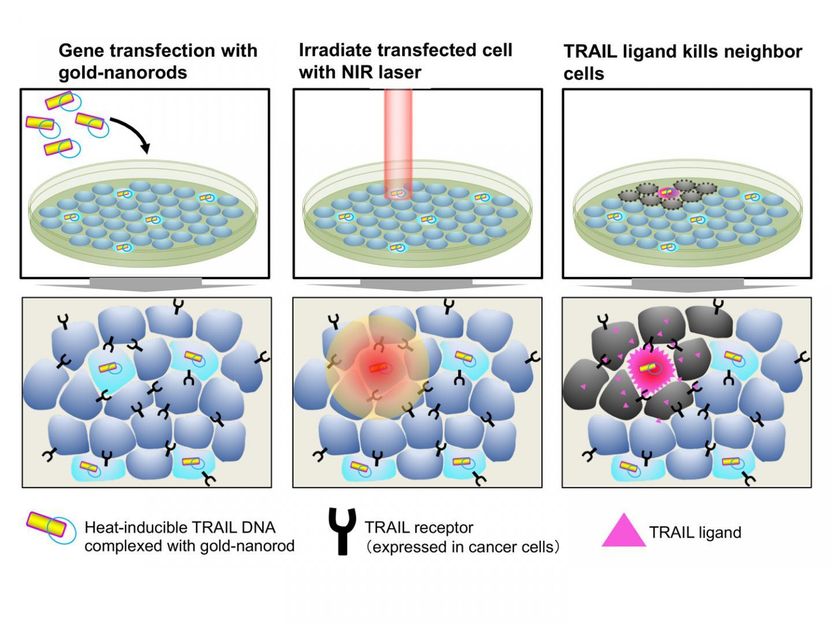
Gold nanorods carrying the heat-inducible TRAIL gene are transfected into cancer cells. Cancer cells express TRAIL receptors while normal cells do not. Illumination by a near-infrared laser warms gold nanorods and induces TRAIL expression in transfected cells. TRAIL then kills the surrounding cancer cells.
Kyoto University iCeMS
The method involves coating gold nanorods, which produce heat when exposed to a near-infrared laser, with the lipids oleate and DOTAP. The lipids enhance the nanorods' ability to interact with and penetrate cells.
The team also developed a gene carrier, known as a plasmid vector, which includes a 'heat shock protein' that is activated in response to heat.
First, the vector was bound to the 'enhanced green fluorescent protein' (EGFP) gene, and then transferred into mammalian cells by the lipid-coated gold nanorods. Exposing cells to near-infrared laser for ten seconds heated up the gold nanorods, turning on the EGFP gene. Surrounding, non-targeted cells showed little to no EGFP expression.
A protein called TRAIL was then added to the plasmid vector. TRAIL induces cell death in cancer cell lines. Infrared illumination of cells transfected by TRAIL-carrying nanorods led to a high cell death rate in surrounding cancer cells.
The lipid-coated gold nanorods could potentially help with molecular cancer therapies.
This new system "provides a unique opportunity for site-directed, light-inducible transgene expression in mammalian cells by a near-infrared laser, with minimal phototoxicity," conclude the researchers in their study.























































