How are long strands of DNA packed into tiny cells?
Scientists are a step closer to understanding how DNA, the molecules that carry all of our genetic information, is squeezed into every cell in the body. How DNA is "packaged" in cells influences the activity of our genes and our risk for disease. Elucidating this process will help researchers in all areas of health care, from cancer and heart disease, to muscular dystrophy and osteoarthritis.

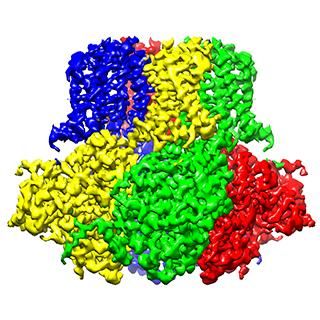
Scientists are a step closer to understanding how our DNA is squeezed into every cell in the body. They provide the first-ever detailed picture of the nucleosome, the most basic building block of chromosomes (the structures that house our DNA). This finding will inform research on all processes that involve chromosomes, such as gene expression and DNA repair, which are critical to the understanding of diseases such as cancer.
University of Rochester Medical Center
DNA is a long, floppy molecule, and there's more than three feet of it in every cell. Our DNA is housed in structures called chromosomes, which condense the DNA to fit into the cell's tight quarters.
Scientists from the department of Biochemistry and Biophysics at the University of Rochester School of Medicine and Dentistry worked with colleagues in France and Japan to describe the first step of DNA packing in a cell. They provided the first-ever detailed picture of the most basic building block of chromosomes, known as the nucleosome, and found that a protein known as H1 (for linker histone H1) helps DNA become more compact and rigid within the nucleosome. In contrast, when H1 isn't present, the DNA is loose and flexible.
The tight structure that H1 creates helps shield our DNA from various factors that can activate or "turn on" certain genes. Without H1, DNA is more accessible to factors that could trigger disease-causing genes.
These finding will inform research on all processes that involve chromosomes, such as gene expression and DNA repair, which are critical to the understanding of diseases such as cancer, according to Jeffrey J. Hayes, Ph.D., senior study author and the Shohei Koide Professor and chair of the department of Biochemistry and Biophysics.
The teams in France and Japan used specialized microscopes and X-rays to capture pictures of DNA molecules interacting with H1 and other key proteins. Because of the size of the DNA and protein molecules, the pictures generated by these techniques were fuzzy and difficult to analyze.
Lead study author Amber Cutter, a graduate student in Hayes' lab, put all of the components -- DNA, H1, and other proteins -- together in tiny test tubes and conducted various biochemical experiments. Her tests, coupled with the X-ray images, confirmed H1's role.
Cutter, who is entering her fifth year in Hayes' lab, admits that the science is complex and that a lot more research needs to be done before this work can inform clinical treatment. But, the importance of understanding the most basic biological processes should not be underestimated. "In order to determine what happens when things go wrong in diseases like cancer, we need to know what happens when things go right."
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

A further step towards decoding smell - Researchers elucidate the role of individual brain neurons in human odor perception
Microtomography
Fascia
Pharmaconomy
Melia_azedarach
Artery_of_Adamkiewicz
Sinus_venosus
Lenalidomide
Thermoreceptor
Torsten_Wiesel

BioStore™ Automated Cryogenic Storage System | Laboratory freezers | Azenta