E. coli bacteria's defense secret revealed
By tagging a cell's proteins with fluorescent beacons, Cornell researchers have found out how E. coli bacteria defend themselves against antibiotics and other poisons. Probably not good news for the bacteria.
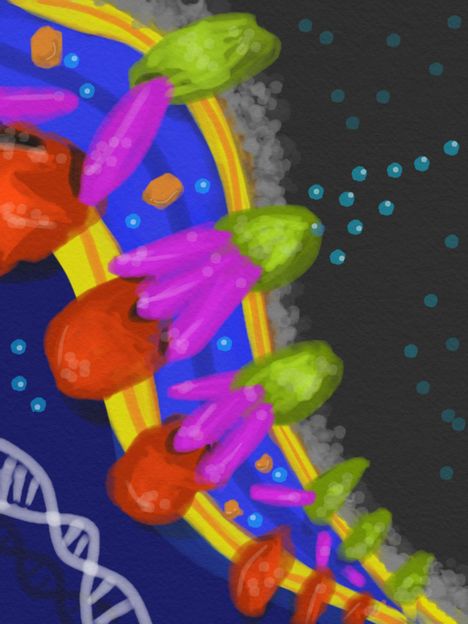
In the periplasm -- the space between the inner and outer membranes of a bacteria's cell wall -- defensive proteins that detect a poison assemble like barrel staves to form a tunnel between pumps in the cell's inner and outer membranes to eject the intruders. Artist's conception by Ace George Santiago.
Ace George Santiago, Cornell University
When undesirable molecules show up, the bacterial cell opens a tunnel though its cell wall and "effluxes," or pumps out, the intruders.
"Dynamic assembly of these tunnels has long been hypothesized," said Peng Chen, professor of chemistry and chemical biology. "Now we see them."
The findings could lead to ways to combat antibiotic-resistant bacteria with a "cocktail" of drugs, he suggests: "One is to inhibit the assembly of the tunnel, the next is to kill the bacteria."
To study bacteria's defensive process, Chen and colleagues at Cornell selected a strain of E. coli known to pump out copper atoms that would otherwise poison the bacteria. The researchers genetically engineered it, adding to the DNA that codes for a defensive protein an additional DNA sequence that codes for a fluorescent molecule.
Under a powerful microscope, they exposed a bacterial cell to an environment containing copper atoms and periodically zapped the cell with an infrared laser to induce fluorescence. Following the blinking lights, they had a "movie" showing where the tagged protein traveled in the cell. They further genetically engineered the various proteins to turn their metal-binding capability on and off, and observed the effects.
The Cornell researchers also collaborated with scientists at the University of Houston, the University of Arizona and the University of California, Los Angeles.
The key protein, known as CusB, resides in the periplasm, the space between the inner and outer membranes that make up the bacteria's cell wall. When CusB binds to an intruder - in this experiment, a copper atom - that has passed through the porous outer membrane, it changes its shape so that it will attach itself between two related proteins in the inner and outer membranes to form a complex known as CusCBA that acts as a tunnel through the cell wall. The inner protein has a mechanism to grab the intruder and push it through.
The tunnel locks the inner and outer membranes together, making the periplasm less flexible and interfering with its normal functions. The ability to assemble the tunnel only when needed, rather than having it permanently in place, gives the cell an advantage, the researchers point out.
This mechanism for defending against toxic metals may also explain how bacteria develop resistance to antibiotics, by mutating their defensive proteins to recognize them. Similar mechanisms may be found in other species of bacteria, the researchers suggested.
Original publication
Other news from the department science
These products might interest you

Hahnemühle LifeScience Catalogue Industry & Laboratory by Hahnemühle
Wide variety of Filter Papers for all Laboratory and Industrial Applications
Filtration Solutions in the Life Sciences, Chemical and Pharmaceutical Sectors

Hydrosart® Ultrafilter by Sartorius
Efficient ultrafiltration for biotech and pharma
Maximum flow rates and minimum protein loss with Hydrosart® membranes

Hydrosart® Microfilter by Sartorius
Hydrophilic microfilters for bioprocesses
Minimal protein adsorption and high flow rates

Sartopore® Platinum by Sartorius
Efficient filtration with minimal protein adsorption
Reduces rinsing volume by 95 % and offers 1 m² filtration area per 10"

Polyethersulfone Ultrafilter by Sartorius
Reliable filtration with PESU membranes
Perfect for biotechnology and pharmaceuticals, withstands sterilisation and high temperatures

Polyethersulfone Microfilter by Sartorius
Biotechnological filtration made easy
Highly stable 0.1 µm PESU membranes for maximum efficiency

Sartobind® Rapid A by Sartorius
Efficient chromatography with disposable membranes
Increase productivity and reduce costs with fast cycle times

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Felicitex Therapeutics and Selvita Initiate Strategic Collaboration to Target Cancer Quiescence

Faecal Pollution: DNA Uncovers Culprit - CSI and forensics can be used to uncover not only serial killers but also the cause of water pollution
Chinese_Mental_Health_Association
Catalent Invests $7.3 Million in Italy - Expands Softgel Encapsulation and Packaging Capabilities in Support of Consumer Health

Bioluminescence - the natural glow - How glowing molecules can also be used in industry
2006 Life Science Industry Awards Finalists
Category:National_Institutes_of_Health_images
Energy sensor as potential target for cancer drugs identified
VaxGen Raises USD 79M Through Sale of Interest in Overseas Biopharmaceutical Manufacturing Facility