One step closer to crack the mystery of bacterial adaptation to antibiotics
An international team including researchers from MIPT's Laboratory for Advanced Studies of membrane proteins have proposed an explanation of the way bacteria process external signals. By identifying the detailed structure of the protein complex used by bacteria, the scientists gained insights into the ability of these microorganisms to detect even small changes in the environment and adapt to them.
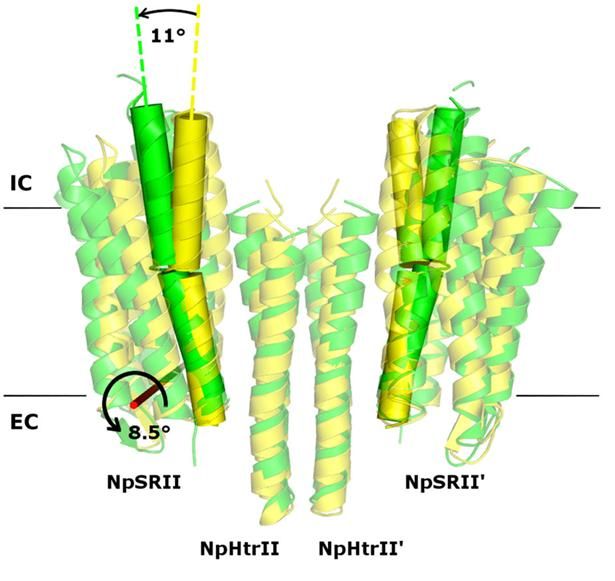
Twofold structure of the protein complex of bacterial sensory photoreceptor rhodopsin II (SRII) and its cognate transducer (HtrII) with the U- and V-conformation shown in yellow and green, respectively.
MIPT Press Office
Bacteria are extremely good at adapting to the changing environment. This renders many antibiotics ineffective, as the bacterial cell can adapt by developing resistance. Because resistant bacteria can survive the influence of drugs, infectious diseases may be difficult to treat.
To gather information about the outside world, bacteria rely on two-component signal systems constituted by transmembrane protein complexes, i.e., structures made up of two proteins in the cell membrane, one of them "sticking out" and the other protruding on the inside. To understand the mechanism behind the operation of such complexes, we need to determine their precise structure. Knowing how this system works, scientists could then figure out a way to switch it off. This makes membrane protein complexes potentially useful targets for emerging antibiotics.
In their study, the researchers examined the crystal structures of the ground and active state of one of such systems consisting of the sensory photoreceptor rhodopsin II coupled with its cognate transducer. The team has demonstrated that this complex can have a U-shaped structure in addition to the regular V-shaped conformation reported in prior research. The study also explains why this is the case by positing biological relevance of the U-shape in terms of signal transduction.
The team suggested that by transitioning from the U- to the V-shape, the receptor-transducer complex enters its active state, which could be involved in signal transmission between the photoreceptor and the transducer. This is in line with existing biological data. Therefore, it is possible to disrupt signal transduction by treating the cell with a suitable drug preventing the V-to-U transition.
"Our findings have a practical application in dealing with bacterial resistance. Nevertheless, this study is primarily significant for our fundamental understanding of signal transduction mechanisms in bacteria, because they could be involved in thousands of other similar bacterial receptors that are responsible for all kinds of cell functions. These insights will enable us to come up with receptor models that are more precise," says Valentin Borshchevskiy, a senior research scientist at the Laboratory for Advanced Studies of Membrane Proteins.
Among other bacterial receptors, the protein complex investigated in the study plays a major role in the aspartate (Tar) and serine (Tsr) receptors. The former guides bacteria towards nutrients (e.g., aspartate and maltose) and away from harmful agents (e.g., nickel and cobalt). The latter is used by bacteria like Salmonella and E. coli to seek serine, which they consume as a nutrient, and avoid harmful acids.
Original publication
Most read news
Original publication
A. Ishchenko, E. Round, V. Borshchevskiy, S. Grudinin, I. Gushchin, J. P. Klare, A. Remeeva, V. Polovinkin, P. Utrobin, T. Balandin, M. Engelhard, G. Büldt & V. Gordeliy; "New Insights on Signal Propagation by Sensory Rhodopsin II/Transducer Complex"; Scientific Reports; 2017
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Membrane vesicles released by bacteria may play different roles during infection
Deutsche_Medizinische_Wochenschrift
Nominations for the European Inventor Award 2010 - Saving precious water, gentler cancer treatments and "football" molecules made of carbon

How to find marker genes in cell clusters - New method facilitates identification of cell-type specific genes in single-cell data

Prepared for the next virus pandemic - 13 institutions from 6 countries set up European research platform
Category:Neurosteroids

Diamond dust shines bright in Magnetic Resonance Imaging - Potential alternative to widely used contrast agent gadolinium

New Partnership of CELLphenomics and Kyan Therapeutics - This partnership aims to offer an efficient approach to expedite drug development process in the biopharmaceutical industry