Breast milk as a weapon against cancer
Advertisement
In a project supported by the Austrian Science Fund FWF, scientists at the University of Graz modified and reinforced a host defence peptide from breast milk so as to enable it to specifically detect cancer cells. The active substance can then induce cell death. Research continues on this new therapeutic approach to types of cancer with limited treatability.
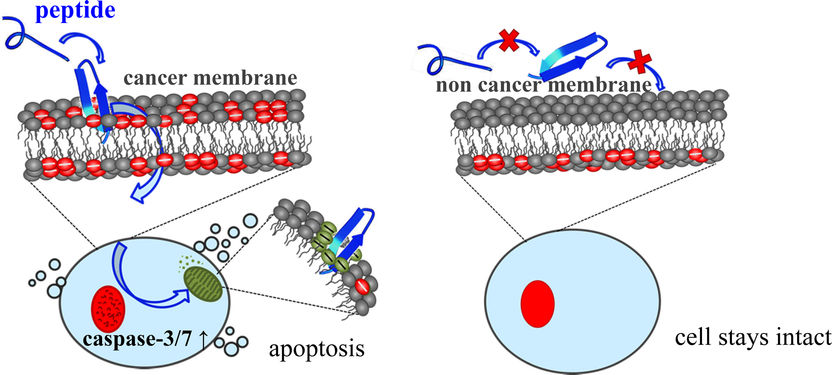
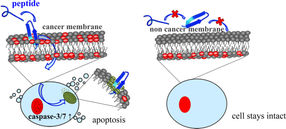
The anti-tumour peptide (blue) interacts with the negatively charged PS markers (red) on the outer membrane of the cancer cell. The peptide is absorbed into the cancer cell, reacts with the mitochondria (green) and induces the controlled self-destruction of the cancer cell (apoptosis). The peptide does not interact with the membrane of healthy cells.
Sabrina Riedl/IMB, Uni Graz
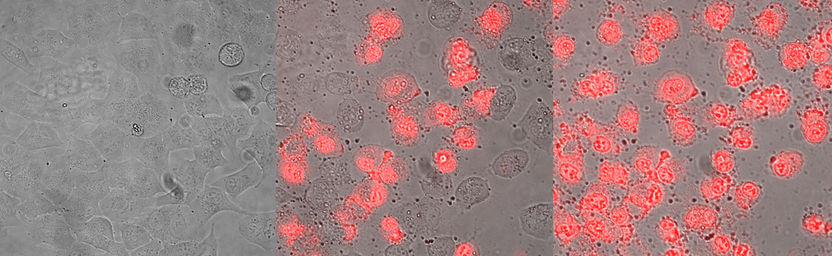
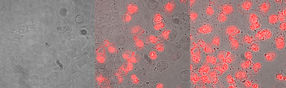
Skin cancer cells before (left), after 4 hours (middle) and 8 hours (right) of treatment with the anti-tumour peptide. The first stage of cancer cell death is blistering (middle), while the increasing uptake of the red dye points to severe membrane damage and final cell death, induced by the peptide treatment (right).
Sabrina Riedl & Dagmar Zweytick/IMB, Uni Graz
Many types of cancer respond well to treatment if detected early and treated with effective chemotherapy. But types that are poorly treatable include skin cancer and brain cancer (glioblastoma) as well as metastases, i.e. tumour cells spreading to remote parts of the body via the bloodstream. But even the most malignant cancer cell has a weak spot, an Achilles heel. Unlike healthy cells, the membrane enveloping cancer cells carries on its outer side negatively charged molecules, the lipid phosphatidylserine (PS). This means that PS can serve as a marker for cancer. With the support of the Austrian Science Fund FWF, a team from the Department of Molecular Biosciences at the University of Graz has developed a new weapon that selectively targets this Achilles heel.
Peptide arrow carved from breast milk
In a preceding FWF project, the team of principal investigator Dagmar Zweytick had already ascertained that the PS cancer marker was an appropriate target for anti-tumour medication, since it was detectable on membranes of various types of cancer and even metastases. In the present follow-up project, the team has now managed to hone a human defence peptide into an arrow that targets cancer cells. A modified and strengthened active part of the peptide lactoferricin, which is found in a precursor in breast milk, identifies various types of cancer cells, including melanoma and glioblastoma. The positively charged peptide arrows selectively locate the negatively charged PS-exposed on the surface of cancer cells, dock on to them and within a few hours induce cell death (a command that is otherwise blocked in cancer cells). The peptide variants do not, however, interact with healthy cells of the body.
Killing cancer without harming healthy cells
“The biggest challenge in the design process was to find the right balance of toxicity and specificity. If the peptides are made too active, they will also attack healthy cells. In control tests we always made sure that the peptides locate only cancer cells and leave normal cells untouched”, explain principal investigator Dagmar Zweytick and postdoc researcher Sabrina Riedl. The biophysicist has been working on lactoferricin since 2002. A precursor of the small protein molecule is present in breast milk, a substance that not only nourishes a new-born child but also helps it build a strong antimicrobial defence system. As part of the innate immune system, lactoferricin is the body’s first defence reaction against negatively charged non-host cells such as bacteria and fungi, but also against mutated body cells, such as cancer cells.
Before the defence peptide can be used as an anti-tumour weapon, it has to be rebuilt in a directed manner to be improved in its activity. Between July 2012 and June 2016, a team of five members worked on the ideal arrangement of the chemical building blocks (amino acids) of the active peptide constituent which is shaped like a hairpin. The team started the design process by simulating variants of promising activity levels in a computer model. About fifteen variants of the substance were synthesised on the basis of these designs and then tested on membrane models and in vitro on cancer cell lines.
Tumour regression in the mouse model
In collaboration with Beate Rinner from the Medical University of Graz, the two most auspicious variants were tested in vivo. The researchers compared peptide-treated and untreated mouse xenotransplants (mice growing human cancer cells). In the cancer mice treated with peptide, the researchers observed a strong or even complete regression of the tumours, achieving an average of 85% regression in the case of melanoma and up to 50% in the case of glioblastoma when compared with untreated cancer mice. A third control group of healthy mice remained unharmed when treated with the agent. The modified variants showed an effectiveness about ten times greater than the original breast milk peptide.
The active peptide variants with an anti-tumour effect have already been patented in the EU and the USA (respectively are patent pending). The team around Dagmar Zweytick is now collaborating with a pharmaceutical company in the context of a project financed by the Austrian Research Promotion Agency FFG in preparation of pre-clinical studies. The new anti-tumour agent should preferably be administered intravenously so as to reach metastases as well. Issues being currently tested include how stable the peptide arrow is in the blood system, whether it can cross the blood-brain barrier and how the arrowhead can be sharpened further.
Original publication
Riedl S., Leber R., Rinner B., Schaider H., Lohner K. and Zweytick D.; „Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine“; Biochim. Biophys. Acta; 1848 (11) (2015) 2918-2931.
Riedl S., Rinner B., Schaider H., Lohner K. and Zweytick D.; „Killing of melanoma cells and their metastases by human lactoferricin derivatives requires interaction with the cancer marker phosphatidylserine“; Biometals; 27 (5) (2014) 981-997.
Riedl S., Rinner B., Asslaber M., Schaider H., Walzer S., Novak A., Lohner K. and Zweytick D.; "In search of a novel target – Phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy"; Biochim. Biophys. Acta; 1808 (2011) 2638-2645.