Membrane lipids hop in and out of rafts in the blink of an eye
New fluorescent lipids demonstrate how specialized regions in the cell membrane function
Researchers in Japan, India and France have found that molecules move into and out of a specialized region of the cell membrane, called the 'raft domain', at unexpectedly fast rates. The discovery was made possible by developing fluorescent compounds that are structurally similar to a special class of lipids called sphingomyelins, and by using a home-built fluorescent microscope sensitive enough to detect single fluorescent molecules.
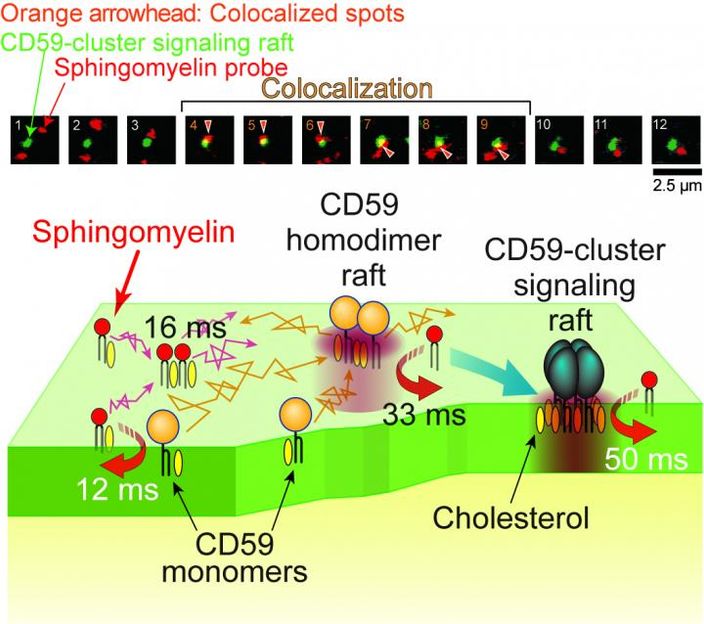
Top: Typical image sequences (every 33 milliseconds) of a raft domain undergoing signaling (green) and a sphingomyelin analog (red). The sphingomyelin analog molecule was associated with a signaling raft domain from frame 4 to 9 (orange arrows). Bottom: Schematic model showing the transient recruitment of sphingomyelins to various raft domains in the time scale of 10 to 50 milliseconds. The signaling raft itself was stable for up to several tens of minutes, working as a stable signaling platform.
Kyoto University iCeMS
Sphingomyelins are key molecules for the signalling functions and the formation of raft domains. But how they mediate the domains' signalling functions was unknown.
A team of researchers led by Akihiro Kusumi and Kenichi Suzuki of Kyoto University's Institute for Integrated Cell-Material Sciences (iCeMS) developed fluorescent compounds that are structurally similar to sphingomyelins and behave in a similar way. To make these fluorescent 'analogs', they attached a hydrophilic (water-preferring) fluorescent molecule to the 'head' part of synthetic sphingomyelin, with a hydrophilic linker group between them, without modifying a critical positive charge in the head group. This arrangement ensured that the fluorescent molecule was placed away from the interior of the cell membrane and did not interfere with the function of the sphingomyelin it was attached to or with the function of the cell membrane.
When the fluorescent sphingomyelin analogs were incorporated into the cell membrane of live mammalian cells in culture, they continually and very dynamically entered and exited from the cell membrane's raft domains. Raft domains are extremely small, around 1/1,000th of the width of a human hair. But they occupy 10 to 20% of the cell membrane area. Quite unexpectedly, the sphingomyelins spent only 12 and 50 milliseconds inside the raft domain before and after extracellular immunostimulation respectively. For comparison, the eye blinks in about 400 milliseconds.
Discovering the very short residency times of sphingomyelins in raft domains represents a large paradigm shift in the research field of cell biology, molecular immunology and molecular neuroscience. Previously, most researchers in these fields believed that raft-associated lipids, including sphingomyelins, were stably localized in raft domains.
"The results suggest that the fluorescent sphingomyelin analogs developed here will be extremely useful for studying sphingomyelin interactions with many other raft-associated molecules and raft domains as well as for understanding the mechanisms of cell membrane signalling and of the invasion of various pathogens," the researchers conclude.
Original publication
Masanao Kinoshita, Kenichi G.N. Suzuki, Nobuaki Matsumori, Misa Takada, Hikaru Ano, Kenichi Morigaki, Mitsuhiro Abe, Asami Makino, Toshihide Kobayashi, Koichiro M. Hirosawa, Takahiro K. Fujiwara, Akihiro Kusumi, Michio Murata; "Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs"; JCB; 2017
Most read news
Original publication
Masanao Kinoshita, Kenichi G.N. Suzuki, Nobuaki Matsumori, Misa Takada, Hikaru Ano, Kenichi Morigaki, Mitsuhiro Abe, Asami Makino, Toshihide Kobayashi, Koichiro M. Hirosawa, Takahiro K. Fujiwara, Akihiro Kusumi, Michio Murata; "Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs"; JCB; 2017
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.