Cellular waste management
How animal cells protect themselves against dangerous goods
In two recent papers, scientists Ahmad Fazeli and Ann Wehman from the University of Würzburg have published new insights into waste disposal in animal cells. These findings may help to better understand the molecular mechanisms underlying autoimmune diseases like lupus.
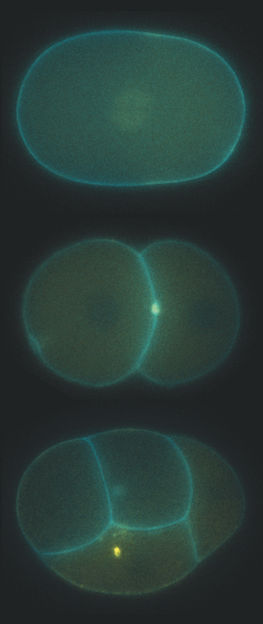
Microscope image of a dividing embryo of the nematode C. elegans. The midbody remnants (yellow) are released as extracellular vesicles, phagocytosed by neighboring cells, and eventually degraded.
Wehman
Animal cells have developed different strategies to degrade unwanted particles. In this way they eliminate invading pathogens, as well as dead cells or cell fragments. Malfunctions in the cell's waste removal systems can lead to overreactions in the immune system or even to autoimmune diseases like lupus.
Scientists from the Rudolf Virchow Center for Experimental Biomedicine at the University of Würzburg investigated a specific type of cell fragment: the midbody remnant. The midbody is a transient structure occurring at the end of each cell division that represents the last linkage between daughter cells. This structure was first discovered in 1891 by the German anatomist Walther Flemming and is therefore also called the Flemming body.
After cell division, the midbody is either inherited by one of the daughter cells or released into the cellular environment. However, in either case, the midbody needs to be rapidly degraded to avoid further impacts on the cell. "Remnants of the midbody can influence the polarity and fate of a cell after cell division”, explains Ahmad Fazeli, first author of the two publications. For example, stem cells and cancer cells accumulate midbodies, which could allow them to give rise to more daughter cells. "However, it was unresolved so far, how cells normally control the removal or degradation of midbodies", continues Fazeli. Previous studies considered two possible scenarios: either the midbody remains inside one of the daughter cells, where it would later be degraded by autophagy (a self-eating mechanism used to remove internal particles), or the midbody is released from both cells to the environment and then taken up again by any cell through a process called phagocytosis (a process used to eat and digest external particles).
In two recent papers, the team of Ann Wehman revealed a model that unifies both possible scenarios, as they describe in the Journal of Cell Science and in Communicative & Integrative Biology. They investigated the midbody in the fast dividing cells of the nematode Caenorhabditis elegans, as many proteins and their cellular functions are similar between worms and humans. They systematically analyzed the roles of different proteins from the phagocytosis and autophagy pathways and discovered unexpectedly that these two pathways can work together in the degradation of the midbody.
As the scientists report, the midbodies in embryonic cells of the nematode are released by daughter cells out to the environment and then eaten by neighboring cells. There, autophagy proteins surround the phagocytosed midbody and help its digestion. This means that proteins responsible for removing internal particles also help to degrade external particles. These recent studies revealed that the midbody is degraded by a process called LC3-associated phagocytosis (LAP). LAP was already known as a sort of cellular waste management process used to remove invading bacteria or the corpses of dead cells. The novel finding is LAP's involvement in midbody degradation.
"At first glance it seems surprising that cells carry out this complex series of events to release the midbody only to take it up again,” explains Ann Wehman, senior author of the study. “However, since this transient structure possesses signaling properties, its regulation is of great importance for the cell." The release of the midbody happens immediately after cell division. Otherwise, a midbody that stays in one of the daughter cells could continue to tell the cell to divide, which could lead to changes in the shape or size of cells or even result in cell fragmentation. Since a midbody could also influence the polarity and fate of the cell that eats it, rapid digestion of this structure is essential. Once the midbody has been broken down into its components, it is rendered harmless and provides the cell with bonus raw materials for further growth.
The new model of midbody degradation reconciles findings of previous studies in nematodes, flies and mammalian cells. Therefore, the authors propose that their findings are likely to be transferable to humans and might help in understanding the mechanisms behind human diseases like cancer or the autoimmune disease lupus.
Currently, the Wehman group is investigating other roles of LAP in developing worm embryos. In this way they want to understand how cells use this process to clean up the cellular environment and protect the embryo from accumulating litter.
Original publication
Fazeli G, Trinkwalder M, Irmisch L, Wehman AM.; "C. elegans midbodies are released, phagocytosed and undergo LC3-dependent degradation independent of macroautophagy"; J Cell Science; 2016; 129(20):3721-3731.
Fazeli G and Wehman AM.; "Rab GTPases mature the LC3-associated midbody phagosome"; Communicative & Integrative Biology; 2017