Supramolecular protein fishing with molecular baits
Advertisement
Scientists from the Center for Self-assembly and Complexity (CSC) successfully isolated a cancer-prone protein by fishing out the proteins using 'molecular bait'. Cancer, according to the American Cancer Society, affects 1 in 2 men and 1 in 3 women, with prostate and breast cancer the most common types afflicting adults. histone deacetylases (HDACs) are an important family of proteins that regulate gene expression; an altered expression or mutations of genes that encode HDACs can induce tumors to develop. Therefore HDACs are among the most promising therapeutic targets for cancer treatment and they have inspired researchers to study and develop HDAC inhibitors. Inhibition of proteins involved in disease is an important strategy in the development of a drug. For example, an anti-cancer drug (SAHA) slows down cancer progression by inhibiting HDAC, which causes the cells to stop reproducing.
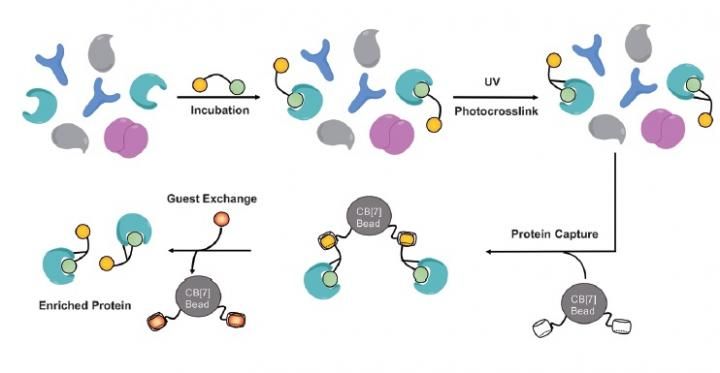
Fig.1: The cell lysate (1.0 mgmL 1) was incubated with SAHA-Ad, and then covalently crosslinked by irradiation with UV light (365 nm). The proteins were precipitated and the free probe removed by methanol washes. The proteins were redissolved and incubated with CB[7] beads for 4 hours at 48C. The beads were washed and the captured proteins were released with BAFc.
IBS
CSC researchers used SAHA as a molecular bait to capture HDAC and successfully fished out the caught proteins using a bead previously developed at the Center. "In 2011, our research group developed a replacement for the existing streptavidin-biotin binding pair, which was used for the separation and purification of proteins, by taking advantage of a strong artificial host-guest interaction pair based on supramolecular chemistry, which was used to capture cell surface proteins with high purification efficiency. The goal of this study was to purify histone deacetylase, a protein that plays a biologically important role in cells and is closely related to disease mechanisms such as cancer," explains Dr. James Murray, the first author of the paper and a researcher from CSC.
The bead is made from Cucurbit[7]urils [CB[7]], a family of pumpkin-shaped macrocycles. These molecules have received a lot of attention because their high-affinity host-guest chemistry provides a unique opportunity to develop non-covalent materials for a range of applications. According to the team's manuscript: "The CB[7]-based enrichment strategy takes advantage of small molecules with exceptionally high binding affinity. In contrast, recombinant methods use larger molecules with lower binding affinity." The IBS team extended this technology to the more complex and function-rich intracellular proteome. Inside the lab researchers labeled a protein of interest with a high-affinity guest for their bead and extracted it from cell lysate using CB[7] beads. The CB[7] beads enriched guest-labeled HDACs from a cell lysate, the team also reported that the method may be useful for enriching proteins labeled from live cells. "We used a drug molecule (SAHA) as the molecular bait that finds HDAC proteins," reveals Dr. Murray. "Our molecule also has a functional group capable of forming a permanent bond with the captured protein when irradiated with ultraviolet light. As a result, it was possible to successfully purify the histone deacetylase from a complex sample."
The team's manuscript went on to clarify, "We demonstrated that affinity-labeled intracellular proteins can be enriched from cell lysates by use of a strong host-guest pair. Notably, this method only uses designed, synthetic molecules to perform the labeling and enrichment, rather than using molecules from nature that have inherent biological background reactivity. The molecular bait strategy is one protein labeling method, we believe that the CB[7] system may be compatible with others too. Furthermore, the CB[7]-system may be used in tandem with conventional Bt-SA enrichments for deep proteome mining. Work along this line is underway in our laboratory."