How proteins reshape cell membranes
Small "bubbles" frequently form on membranes of cells and are taken up into their interior. The process involves EHD proteins - a focus of research by Prof. Oliver Daumke of the MDC. He and his team have now shed light on how these proteins assemble on the surface of a cell and reshape its membrane.
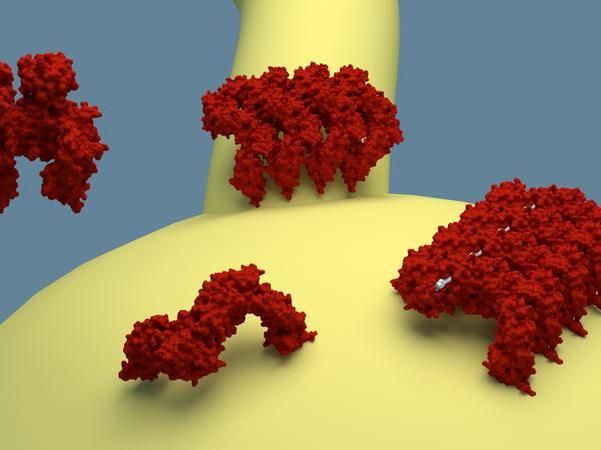
The EHDs present in the cell are in an inhibited state (top left). Once the proteins bind to the membrane, they change to an active state and build chains that can deform the membrane and stabilize tubular deformations, as shown on top.
Artur Alves de Melo, MDC
Fairground entertainers can transform simple balloons into elaborate figures with just a few twists. They do this by "pinching off" sections of the balloon's surface - a method similar to that used by cells to create small bubbles known as vesicles for the transport of molecules. Vesicles are used to take up nutrients and play an important role in the transmission of neural signals.
EHD proteins are one type of molecular machine responsible for the creation of vesicles. These proteins bind themselves to the inside of a cell membrane, where they form long chains and ring-like structures. The rings then invaginate the membrane, contract like a drawstring, and, finally, detach the vesicle from the surface of the cell.
Oliver Daumke of the Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) is investigating the function and spatial structure of these EHD proteins. In an earlier study, he and his team analyzed the three-dimensional structure of EHDs in an inactive form - i.e. not bound to the membrane. Until now, scientists did not know how EHD proteins become activated, attach to the membrane and shape it into tubular structures.
Together with international colleagues, Daumke and his PhD student Arthur Alves de Melo have now published an article that describes the active form of this molecular EHD machine - that which occurs when it comes into contact with the membrane. Comparing the active and inactive protein structures, they discovered that EHD molecules flip open when they bind to the membrane, exposing specialized regions. One of these regions allows them to organize in extended chains and ring-like structures. Another region reorients toward the membrane and anchors the molecular machines on the cell surface.
With this work, Daumke's team has now described two steps in the operation of EHDs. "To understand the complete operating cycle and thus the full function of these molecular EHD machines, we must now analyze various other states," he says. "That is a task for the coming years."
Original publication
Other news from the department science
These products might interest you

Hahnemühle LifeScience Catalogue Industry & Laboratory by Hahnemühle
Wide variety of Filter Papers for all Laboratory and Industrial Applications
Filtration Solutions in the Life Sciences, Chemical and Pharmaceutical Sectors

Hydrosart® Microfilter by Sartorius
Hydrophilic microfilters for bioprocesses
Minimal protein adsorption and high flow rates

Hydrosart® Ultrafilter by Sartorius
Efficient ultrafiltration for biotech and pharma
Maximum flow rates and minimum protein loss with Hydrosart® membranes

Sartobind® Rapid A by Sartorius
Efficient chromatography with disposable membranes
Increase productivity and reduce costs with fast cycle times

Polyethersulfone Microfilter by Sartorius
Biotechnological filtration made easy
Highly stable 0.1 µm PESU membranes for maximum efficiency

Sartopore® Platinum by Sartorius
Efficient filtration with minimal protein adsorption
Reduces rinsing volume by 95 % and offers 1 m² filtration area per 10"

Polyethersulfone Ultrafilter by Sartorius
Reliable filtration with PESU membranes
Perfect for biotechnology and pharmaceuticals, withstands sterilisation and high temperatures

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.