Study finds 'sweet spot' where tissue stiffness drives cancer's spread
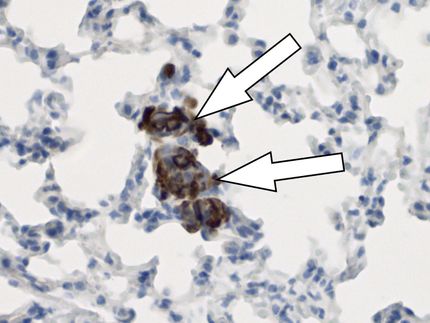
In order for cancer to spread, malignant cells must break away from a tumor and through the tough netting of extracellular matrix, or ECM, that surrounds it. To fit through the holes in this net, those cancerous cells must elongate into a torpedo-like shape.
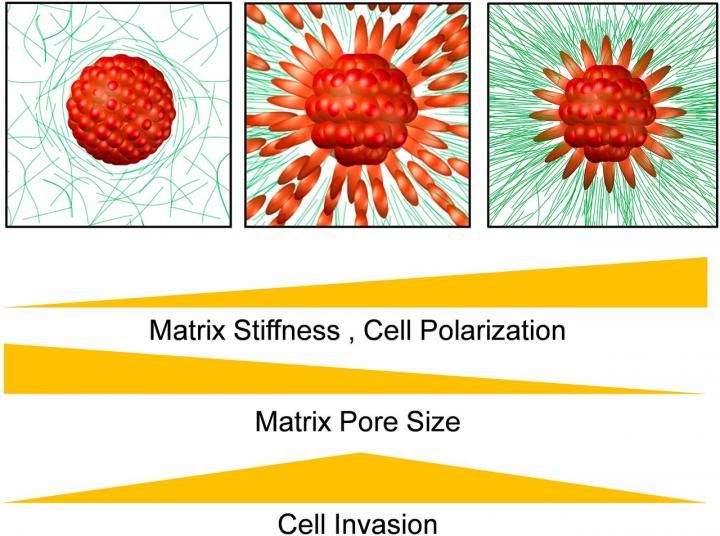
Cancer cells respond to an optimal stiffness of surrounding ECM. If the collagen is too soft, it doesn't pull them hard enough to break cell-cell adhesion and leave the tumor. If it's too stiff, the pores in the ECM are too narrow for the cells to escape.
University of Pennsylvania
Researchers from the University of Pennsylvania and The Wistar Institute have now found that physical forces exerted between these cells and the ECM are enough to drive this shape change. Those forces converge on an optimal stiffness that allows cancer cells to spread.
The findings suggest that drugs that target the stiffness of the ECM could potentially be used to prevent metastasis.
he study was led by Vivek Shenoy, professor in the Department of Materials Science and Engineering in Penn's School of Engineering and Applied Science, and Hossein Ahmadzadeh, a graduate student in his lab, with contributions from Ashani Weeraratna, the Ira Brind Associate Professor and program leader of the Tumor Microenvironment and Metastasis Program at Wistar.
Research on the physical-feedback mechanisms between cancer cells and their environment is part of Penn Engineering's larger efforts to understand such dynamics, housed at the Physical Sciences Oncology Center and the new Center for Engineering Mechanobiology, which is co-directed by Shenoy.
Shenoy and colleagues published findings that detailed the feedback mechanism exhibited by cancer cells and the ECM surrounding them. There, they showed how this mechanism stiffens and aligns the collagen fibers found in ECM. The new work looks at the cell side of the equation and how cells must switch from rounded to elongated in order to leave the tumor squeeze through the ECM.
"What we're showing is that the mechanical factors alone can lead to the change in phenotype in cancer cells," Shenoy said. "This is the first quantitative analysis of the shapes of cancer cells as they invade from the tumor."
The Penn researchers postulated that the key factor of this interplay is finding a "sweet spot" in the stiffness of the ECM.
"The cells in a tumor are sticky," Shenoy said. "Without the collagen fibers of the ECM pulling on those cells, you can't break that cell-cell adhesion. But, if the ECM is too stiff, the pores in the matrix become too narrow and the cells can't escape."
After the Penn team modeled these interactions in computer simulations, the Weeraratna lab at Wistar conducted matching experiments to see if the results held up.
"We used melanoma spheroids embedded in a collagen matrix as a 3-D model to mimic in vitro what happens in the body when tumor cells leave the primary tumor to invade other tissues," said Weeraratna. "Our observations perfectly matched and complemented the computer model. This study reaffirms, from a mechanobiology standpoint, the crucial role of the tumor microenvironment in orchestrating the fate of cancer cells and dictating prognosis and response to therapy."
Insights from cancer mechanobiology could inform future diagnostics and potentially even treatments.
"The takeaway is that, if you look at what's going on outside the tumor, you could make a prognosis of whether it will spread," Shenoy said.