Know your enemy
An arsenal to fight antibiotic-resistant bacteria
Advertisement
To fight your enemies, it helps to know their weaknesses. And, the more specific your knowledge, the easier it is to undermine their defenses. If your enemy sits safe behind a giant wall, for example, its valuable to know how your foe constructed it, what materials they used, and what cracks you could exploit.
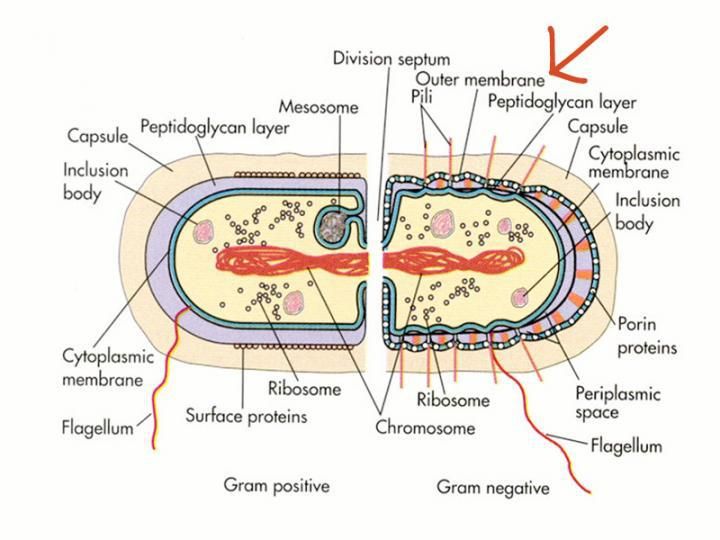
Gram-negative bacteria's thick outer membrane prevents entry of toxic molecules, including antibiotics.
Microbiology Concepts (http://microbiologyconcepts.blogspot.com/2017/03/bacteria.html)
We share a global enemy: antibiotic-resistant bacteria. According to the Centers for Disease Control and Prevention (CDC), "without urgent action, many modern medicines could become obsolete, turning event common infections into deadly threats." Certain bacteria, so-called Gram-negative bacteria, have a thick outer defense that protects them from toxins, antibiotics included.
To combat this growing crisis, researchers in the Kahne Lab meticulously document how our enemies work. During the last several years, they identified a number of previously unknown molecular machines and processes that build the bacteria's stubborn barrier, called the outer membrane. With these discoveries, they're starting to unravel its weaknesses.
Now Professor Daniel Kahne along with postdoctoral researcher Ran Xie and graduate student Rebecca J. Taylor, describe their latest findings. Gram-negative bacteria build their outer membrane, they write, with a burly glycolipid called lipopolysaccharide (LPS). So, if we could prevent LPS from reaching the outer membrane, their defense could weaken.
"To understand the factors that influence LPS transport, we developed a quantitative method to monitor transport rates," the authors report. Previously, the team designed a system to understand how LPS transport occurred. Most recently, they used a fluorescent material that binds to LPS to measure how much, and how quickly, it accumulates in the outer membrane. In addition, the team used their fluorescence-based test to learn which molecular components are integral to LPS transport. If, for example, the bacteria rely on one machine to build their barrier, researchers could investigate how to dismantle the machine and, therefore, the defense.
With their novel fluorescence-based tool, the team discovered crucial new details about LPS transport. ATP hydrolysis--the process by which the cell produces energy--is in fact integral to LPS transport. If transport stops, ATP hydrolysis stops. In addition, even if the cell has LPS in reserve and energy to spare, it will still stop transport. The team determined that the translocon--the machine that carries the LPS across the cell's membranes--controls the movement. "Using mutants of the transport machinery, we find that the final amount of LPS delivered into the membrane depends on the affinity of the outer membrane translocon for LPS."
Of course, more research is required to understand how the molecular machines and mechanisms in Gram-negative bacteria function (and malfunction). But the Kahne Lab's investigative work could one day lead to new treatments to combat antibiotic resistance and save lives, globally.

























































