In the fight against Alzheimer's, Down syndrome may hold vital clues
At first glance, Down syndrome (DS) and Alzheimer's disease (AD), two severe brain abnormalities, may seem to have little in common. Down syndrome is a hereditary disease, the source of which has long been recognized--a triplication of chromosome 21. By contrast, the overwhelming majority of Alzheimer's cases (over 95 percent), do not have a clear-cut genetic source. Instead, the disease, which usually becomes clinically apparent late in life, is caused by a perplexing constellation of factors. While these have been the focus of intense study for over 100 years, few conclusive answers have come to light.
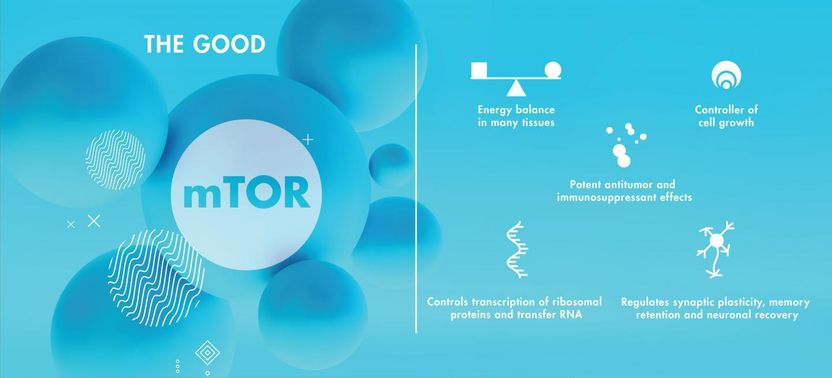
mTOR is a protein complex playing many essential roles in the body.
Shireen Dooling
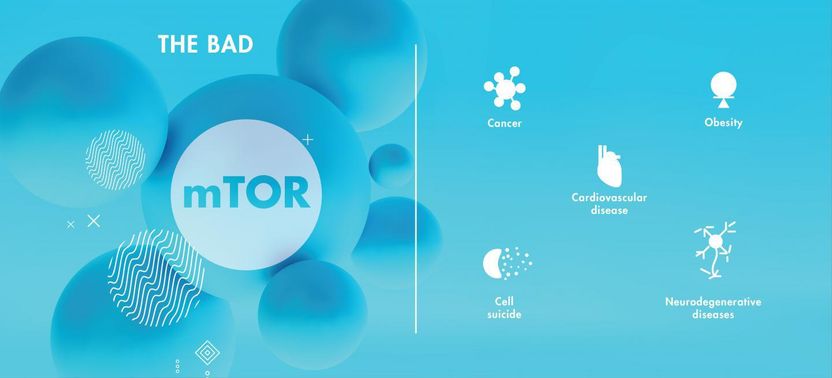
Hyperactivity of mTOR has been associated with a number of adverse effects and is implicated in the neurodegeneration associated with both Down syndrome and Alzheimer's.
Graphic by Shireen Dooling


In new research, Antonella Caccamo and her colleagues explore a number of critical factors that appear to link the two illnesses. The current project will use DS as a window into the underlying mechanisms that may give rise to Alzheimer's pathology. Using this complementary approach, her $3.1 million NIH grant will explore the effects of a critical protein complex known as mTOR.
In the healthy brain, mTOR is involved in a range of essential physiological processes. mTOR is a regulator of protein synthesis and degradation. It plays a critical role in cell growth, longevity and the formation of the cytoskeleton, which provides living cells with their shape and structure, and mTOR is vital to maintaining the proper energy balance in many tissues throughout the body. mTOR is also implicated in synaptic plasticity, neuronal recovery and the retention of memory.
Caccamo is a researcher in the ASU-Banner Neurodegenerative Disease Research Center. Much of her research focuses on investigating Down syndrome molecular alterations in the brain in order to shed new light on Alzheimer's disease.
"The ultimate goal of my research is to identify novel and clinically translatable targets, thus aiding in the development of new treatments for AD," Caccamo says.
Learning from mTOR
Disruption of the mTOR pathway has been implicated in diseases including cancer, obesity and cardiovascular disease. Dysregulation of mTOR also plays an important role in diabetes and aging, two known risk factors for Alzheimer's disease. Irregularities in mTOR functioning are linked to other neurodegenerative diseases and have been shown to give rise to two distinct neuropathologies: depositions in the brain of plaques composed of the protein amyloid beta (Aβ), and accumulations of another protein-- known as tau--which aggregates within neuronal cell bodies, forming neurofibrillary tangles.
Plaques and tangles are the classic hallmarks of Alzheimer's disease. Intriguingly, they also occur in the brains of virtually all patients with Down syndrome, some 60 percent of whom go on to develop Alzheimer's disease by age 60. Interestingly, APP (amyloid precursor protein), a protein that when cleaved generates beta amyloid (Aβ), the toxic protein that accumulates in AD and DS brains, is located on chromosome 21, the same chromosome that is triplicated in Down syndrome.
Could disruption of the vital mTOR pathway offer clues to the development of plaques and tangles and the onset of dementia in both DS and AD patients? Is mTOR dysregulation also linked with a particular form of cell death known as necroptosis, likewise implicated in AD and DS pathology? Most importantly, can the investigation of the molecular drivers of AD pathology in DS patients provide a new window into the early mechanisms underlying the development of sporadic Alzheimer's, the form of the disease that commonly strikes aging adults? These are some of the important questions Caccamo's new study intends to address.
Relentless scourge
Alzheimer's disease remains the only leading killer lacking any means of treatment, prevention or cure. The disease is pitiless in its systematic destruction of brain functioning, wiping memories clean and robbing the brain of its essential capacities, ultimately resulting in death--typically within 8-10 years of clinical diagnosis, though in some cases, Alzheimer's can drag on for as long as 20 years. The emotional toll on patients, caregivers and society is immense and rapidly mounting.
Additionally, the staggering economic burden currently figures in the hundreds of billions of dollars in the US alone and is projected to top $1 trillion by 2050. The need for viable treatments and preventive strategies could not be more acute.
Today, researchers know that the onset of Alzheimer's begins decades before its telltale signs become apparent. Some have gone so far as to say that while AD is usually thought of as a disease of old age, it may also be associated with adolescence when the early signposts of the disease are planted in the seemingly healthy brain. Many in the field believe that the best hope for arresting the ominous trajectory of the disease lies in identifying causal mechanisms at the earliest stage, and developing effective means of intervention before the brain is irreparably damaged.
Caccamo believes that mTOR dysregulation may be one such early mechanism, giving rise to AD pathology in aging adults as well as DS patients. Research has demonstrated that mTOR is hyperactive in specific brain regions in both AD and DS patients. mTOR hyperactivity is further associated with tau pathology as well as low levels of TSC2, a critical gene product that is believed to keep mTOR hyperactivity in check. Finally, preliminary data from Caccamo's research indicates that cell loss in DS patients results in part from necroptosis, a unique form of cell suicide linked with dysregulation of mTOR.
This combination of factors has led to the central hypothesis of the new study: Dysfunction of the TSC2 complex causes an increase in mTOR activity in DS, leading to AD-like neurodegeneration by inducing necroptosis.
Streams and tributaries of Alzheimer's pathology
Caccamo's new project, entitled "Identify common mechanisms of neurodegeneration between Alzheimer's disease and Down syndrome," addresses these issues on several fronts. The first aim of the project is to identify the molecular mechanisms underlying mTOR hyperactivity in DS. Here, the association of dysfunctional TSC2 with mTOR hyperactivity is explored. What might be causing the downregulation of TSC2 leading to mTOR hyperactivity? Three possibilities are experimentally probed: the presence of epigenetic changes in TSC2 and mTOR, alteration of the turnover rate of the TSC2 protein and newly detected proteins that may likewise contribute to destabilizing the delicate TSC2/mTOR axis.
The second aim of the study is to determine the role of hyperactive mTOR in the development of AD-like phenomena in DS. Here, the hypothesis of hyperactive mTOR leading to AD pathologies, particularly Aβ plaques and neurofibrillary tangles, is explored using Ts65Dn mice, a genetic model of Down syndrome. Caccamo's preliminary results show that mTOR hyperactivity precedes an increase in Aβ and tau levels and degeneration of cholinergic neurons in mice. By subtly increasing or decreasing mTOR signaling, the study will test the effects of reducing mTOR on Aβ and tau levels as well as degeneration of neurons in the mice. Further, increased mTOR levels will be examined to see if such changes increase AD-like pathology and cognitive deficits. Finally, the study will identify additional proteins falling under the regulation of hyperactive mTOR in DS.
Although the death of nerve cells in both Alzheimer's and DS brains is a well-recognized occurrence associated with impaired cognitive ability, the mechanisms leading to cell death are still not well understood. The third aim of the new study will be to examine how mTOR hyperactivity contributes to neuronal loss. Earlier work by Caccamo and others suggests that a form of programmed cell death known as necroptosis contributes to the neurodegeneration typically observed in AD brains.
The third phase of the new study will investigate the hypothesis that hyperactive mTOR helps set this neurodegeneration process in motion by activating necroptosis pathways in the brain. Systematically modulating mTOR activity and necroptosis signaling in mouse neurons will be used to test this hypothesis. In addition to improving the understanding of the mechanisms leading to cell death in DS and AD, the research will help elucidate possible therapeutic targets for these two tragic afflictions.
Researchers have much to learn from in-depth studies like these, which delve into mTOR's profound influence on the brain, in sickness and in health. In addition to its relevance in neurodegenerative disease, mTOR's crucial role in the aging process may shed new light on other foundational issues in neuroscience.