Images of NMDA receptors help explain how they are affected by zinc and a drug
The difference between mental health and mental illness can turn on changes in brain cells and their connections that are almost incomprehensibly tiny, at least in physical terms. This irony is brought to light by X-ray crystallography, a method that enables neuroscientists to map the structure of brain proteins atom by atom, using high-energy X-rays.
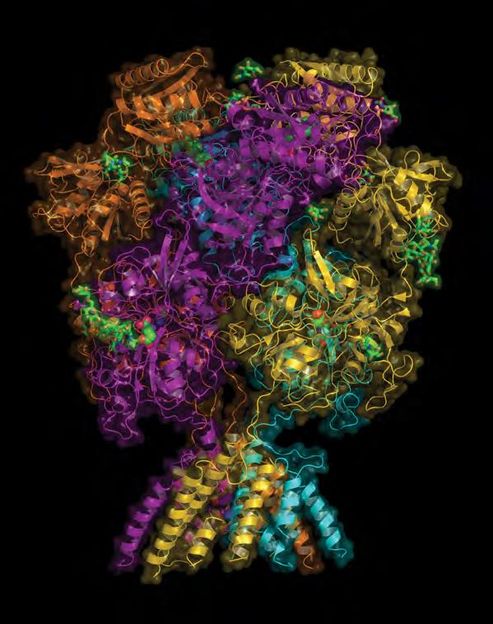
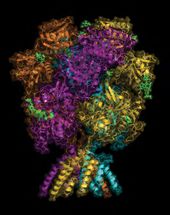
This overall view of the NMDA receptor shows its hot air balloon-like shape. Much of the "balloon" portion protrudes from the surface of glutamate neurons. It engages with signals coming from outside the cell. The "basket" portion the at the bottom lies well below the cell membrane, in the cytoplasm. It forms the terminal end of the receptor's ion channel. NMDA dysfunction is implicated in depression, schizophrenia and Alzheimer's disease, among others.
Furukawa Lab, CSHL
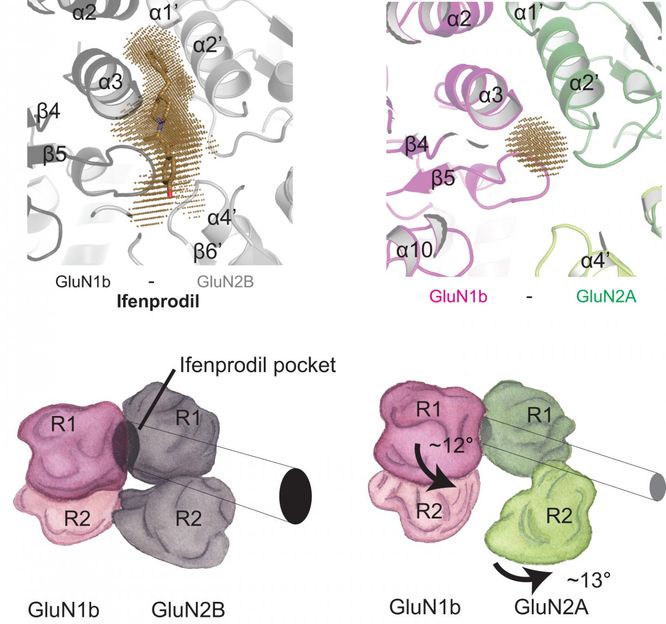
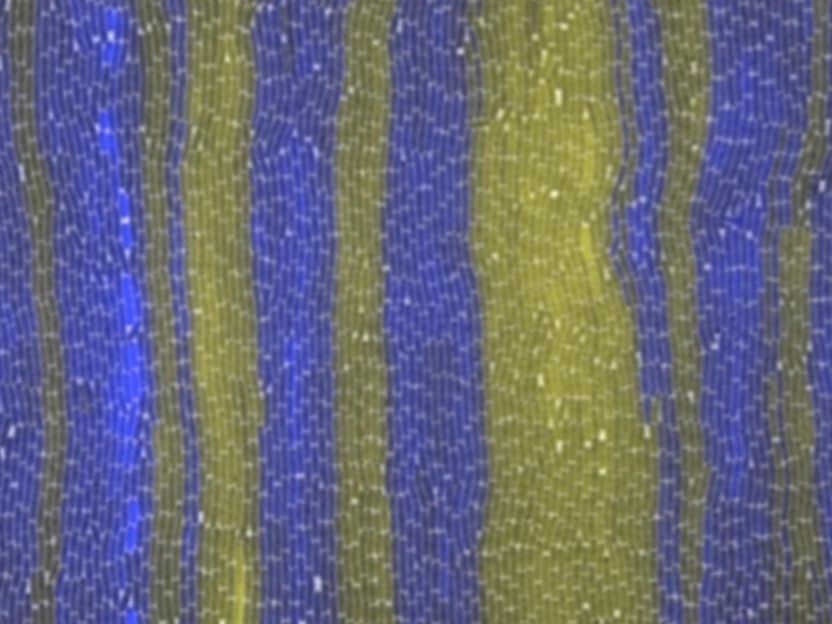
X-ray crystallography by Furukawa and team show how very slight changes in structure between two variants of one of the NMDA receptor's domains can affect drug binding. On the left, a binding pocket for the drug ifenprodil created in the interface between two subdomains of the receptor, called GluN1b and GluN2B, is shown above in brown, and rendered below as it would appear visually. Tiny structural differences in the latter subdomain, in a variant called GluN2A, collapse the space between the two subdomains and prevent ifenprodil from binding. Drugs generally cause fewer side effects when they are extremely specific in their binding sites.
Furukawa Lab, CSHL
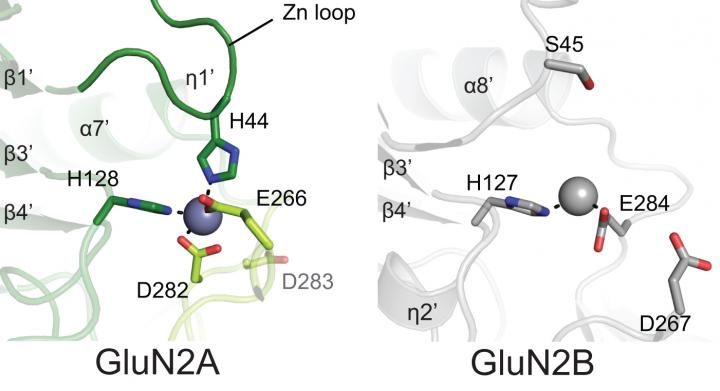
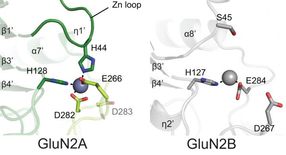
Zinc is an important inhibitory regulator of NMDA activity. It binds more readily and more robustly to the NMDA variant subdomain at left (the GluN2A subunit) than at the same position in the very similar GluN2B subunit. Four amino acids cup the zinc molecule (blue) like four hands in GluN2A vs. two amino acids, or two hands, bonding with it (grey) in GluN2B.
Furukawa Lab, CSHL
In research appearing today in Neuron, a team at Cold Spring Harbor Laboratory (CSHL) reveals for the first time the structure of a portion of an important brain cell receptor, called the NMDA (N-methyl D-aspartate) receptor. The newly mapped portion of NMDA receptor is responsible for recognizing zinc, an element common throughout the brain that can inhibit a class of NMDA receptors.
Large in size compared with many other proteins, NMDA receptors are shaped like hot air balloons, ones that poke through the surface membrane of neurons in the brain that receive glutamate, the most common excitatory neurotransmitter.
NMDA receptors are activated when a glutamate molecule docks at a site on the receptor, setting in motion a complex series of changes. But that is only part of what is happening. Along with glutamate, some sets of neurons co-release zinc, which can also interact with NMDA receptors, playing an important role in controlling neuronal signaling. The binding of zinc to NMDA receptors is also an important factor in the transmission of the sensation of pain.
The precise structure of the NMDA receptor is critical in current efforts to design new and more effective drugs to treat a host of mental disorders. Overactive NMDA receptors are linked with neurodegenerative illness, such as Alzheimer's disease. Underactive NMDA receptors may be a factor in schizophrenia. The receptors are also implicated in depression and epilepsy, among other illnesses.
Different sections, called domains, of the giant NMDA protein are involved in these various illnesses, and they are joined together in four major combinations, or subtypes. The subtypes, referred to by the letters A, B, C and D, have specific functions and appear at different times in life and places in the brain. Specific targeting of those subtypes is important in efforts to minimize side effects of new drugs for brain disorders now being tested in labs and clinical trials.
The subtype revealed in the new research, led by CSHL Associate Professor Hiro Furukawa, is the "A" form of the NMDA receptor. The team mapped the amino terminal domain (ATD), one of the four domains of the GluN2A subunit of the receptor. The ATD -- the outermost part of the NMDA structure, poking out into extracellular space -- is pictured by the team as it forms a complex with zinc. [see illustration]
The team's high-resolution pictures of the "A" type of the NMDA receptor are the product of 3 years of work by Annabel Romero-Hernandez, a talented graduate student in the Furukawa lab studying at the Watson School of Biological Sciences. "Annabel's beautiful new pictures are important for a few reasons," says Furukawa. "They have enabled us to solve two puzzles, both raised by earlier pictures we made of the 'B' type of the receptor. First, why does zinc bind so much more readily to the 'A' form compared with the 'B' form, which is structurally almost identical? And second, why does the important candidate drug ifenprodil bind at a site in the 'B' form but not in the 'A' form?"
What the new images reveal about zinc binding
Furukawa and Romero-Hernandez were intrigued by the zinc molecule's interaction with the 'A' form of the receptor in part because of discoveries in recent years of zinc's abundance and important role in the mammalian brain. When a message-transmitting NMDA receptor releases glutamate, it co-releases zinc, which interacts with the receptor at an alternate binding site, called an allosteric site. In the already-mapped 'B' form of the receptor, comparatively large amounts of zinc had to be present if binding was to occur; and the binding was found to be physically weak.
The new pictures of the receptor's 'A' form show why much less zinc is needed to achieve binding, and explains why the binding is much more robust. Zinc slips into the pivot point in a clamshell-like assembly in the ATD, and atomic-level resolution reveals that in the 'A' form, it is held firmly in place by bonds formed with four surrounding amino acid molecules. In previously obtained views of the 'B' form, only two such amino acids lie at the comparable spot in the ATD's structure. The difference in binding affinity, says Furukawa, "is like the difference between having two hands holding up an object vs. four hands." [see illustration]
Zinc's impact on the function of the much larger full NMDA receptor is profound: its binding to the ATD is a regulator of the receptor' central channel that connects the exterior environment to the cell's interior. Zinc inhibits the ion channel, encouraging it to close up -- much more strongly in the 'A' type than the 'B' type. "Zinc binding is one of the means by which NMDA receptors regulate themselves," says Dr. Romero-Hernandez. Such self-regulation is important, she notes, considering that "when you overactivate these receptors the consequence can be neurodegenerative."
The receptor's ion channel flickers open and closed very rapidly -- in a tenth to a hundredth of a second -- as the brain performs its complex operations. Whether it is being inhibited by zinc or excited by glutamate or other binding partners, the result is profound, considered at the level of entire brain regions.
A drug binding pocket that "collapses"
The same mapping of the ATD in the 'A' form of the NMDA receptor also solved the question of whether ifenprodil can bind at an allosteric site in the ATD identified clearly in the lab's prior work on the 'B' form.
The new pictures obtained by Furukawa and Romero-Hernandez show clearly that in the 'A' form, "the binding pocket, which was so large in the 'B' form, has now collapsed into a much smaller space," says Furukawa. "Now we understand structurally why the drug cannot bind in the 'A' form." This could be crucial to drug developers, since the more specific a drug is, the less likely it will have unwanted side effects.
With recent research demonstrating the almost magical power of the drug ketamine - an NMDA inhibitor -- to alleviate the worst forms of non-responsive major depression in some patients, there is great interest in finding mechanisms underlying the illness that do not involve the neurotransmitter serotonin. So-called SSRI antidepressants (drugs in the Prozac class) are taken by tens of millions, many of whom are not helped by it. Furukawa says his work on the NMDA receptor is aimed in part on exploring alternate hypotheses of depression's causation involving NMDA or glutamate-mediated dysfunctions. His lab is pursuing this work in structural studies as well as by studying signaling pathways inside neurons - activated by the interaction of receptors like the NMDA receptor with the environment beyond the cell membrane.