Rapid synthesis towards optically active alpha-aminocarbonyl therapeutics
New catalytic asymmetric reaction directly installs amines into carbonyl compounds
A team of organic chemists at ITbM, Nagoya University, has developed a new reaction to directly install amines into carbonyl compounds using their unique phase-transfer catalyst. This unprecedented method leads to the rapid formation of optically active (chiral) α-aminocarbonyls, which are structural moieties found in many biologically active compounds and in therapeutics, such as anti-malarial and anti-HIV agents.
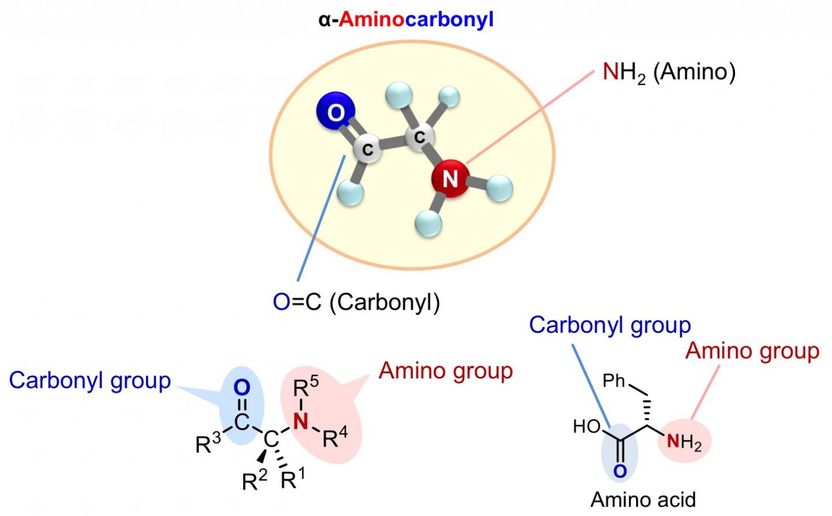
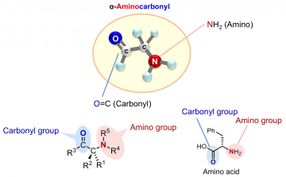
Chiral α-aminocarbonyls are present in a range of biologically active compounds, including amino acids and proteins.
ITbM, Nagoya University
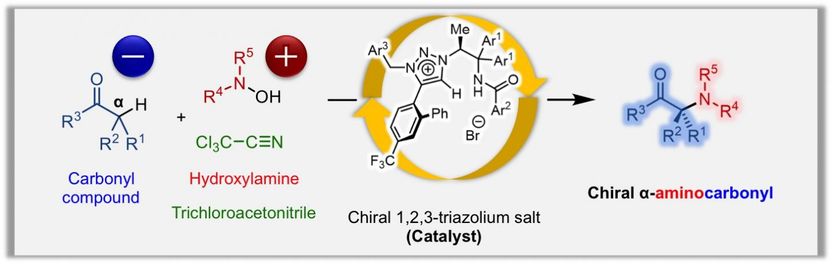
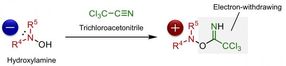
The hydroxylamine is converted into a reactive intermediate in the presence of trichloroacetonitrile. The chiral 1,2,3-triazolium salt, a unique phase transfer catalyst developed by Ooi's group, enables stereoselective carbon-nitrogen bond formation by the generation of chiral enolates of carbonyl compounds.
ITbM, Nagoya University
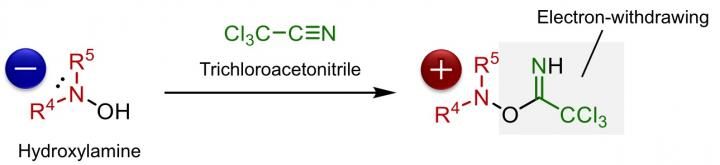
Activation of hydroxylamines by trichloroacetonitrile, making it ready for the reaction with carbonyl compounds. The reaction intermediate has been identified to be the O-iminohydroxylamine (structure on the right hand side).
ITbM, Nagoya University
Nagoya, Japan - Dr. Kohsuke Ohmatsu, Professor Takashi Ooi of the Institute of Transformative Bio-Molecules (ITbM) of Nagoya University and their colleagues have reported on the development of a new chemical transformation that directly introduces amines at the α-carbon position of carbonyl compounds. This new method enables rapid access to chiral α-aminocarbonyls from readily available carbonyl compounds and hydroxylamines.
Chiral α-aminocarbonyls are found in many biologically active compounds such as amino acids and proteins, as well as in various pharmaceuticals.
Many α-aminocarbonyl compounds have a chiral center, where the α-carbon has 4 different groups attached to it. These α-aminocarbonyl compounds can exist as enantiomers. Enantiomers usually have different properties, and intensive studies have been carried out across the world to selectively synthesize a particular enantiomer (enantioselectivity).
Although chiral α-aminocarbonyls have been known to be an essential structural moiety in various biological molecules, the direct catalytic asymmetric introduction of amines into carbonyl compounds to access α-aminocarbonyls has been unexplored up to now. This is due to the repulsion of electrons between the carbon atom and the nitrogen atom, on the enolate of the carbonyl compound and amine, respectively. Ooi's group solved this issue by reacting hydroxylamine with trichloroacetonitrile, which lowers the electron density of hydroxylamine, enabling it to react with various carbonyl compounds.
"We were able to develop a new catalytic asymmetric reaction to directly synthesize chiral α-aminocarbonyls in high enantioselectivity, from carbonyl compounds in one single step, where we used hydroxylamine as an amine source and our original chiral catalyst," says Kohsuke Ohmatsu, an Associate Professor at ITbM. "Although we had this idea in mind from about 3 years ago, it took us a while to identify the right conditions using the appropriate starting materials," he continues. Yuichiro Ando and Tsubasa Nakashima, who are both graduate students in Ooi's group, took up this work.
"The goal of our study was to develop a reaction to make a variety of chiral α-aminocarbonyls from a wide range of carbonyl compounds and hydroxylamines," says Takashi Ooi, a leader of this study. "We tested a variety of carbonyl compounds, but had extreme difficulties at the beginning of our studies to identify the factors that were responsible for enabling the reaction to proceed," he explains. "Indeed, this required a lot of trial and error to figure out what was necessary for the reaction. We challenged ourselves to make difficult situations into opportunities, and kept on analyzing the data objectively to move on to the next step."
"I must say that the best moment for us was when we saw the NMR (nuclear magnetic resonance) spectrum to identify the molecular structure of the desired α-aminocarbonyl product for the first time," describes Ohmatsu. "The key for this reaction was to use trichloroacetonitrile to activate the hydroxylamine, along with our chiral catalyst."
The Ooi group's unique catalyst consists of a chiral 1,2,3-triazolium ion, which has a high hydrogen-bond-donating ability that can activate carbonyl compounds to react with hydroxylamines, which itself is activated by trichloroacetonitrile. This new reaction enables rapid access to chiral α-aminocarbonyls by the direct introduction of amines into carbonyl compounds, a process that has been quite challenging by previous approaches.
"We hope to improve our range of starting materials that we can use in order to access an even wider range of α-aminocarbonyl compounds, which may lead to the rapid generation of molecules with potential therapeutic applications," say Ohmatsu and Ooi. "To do so, we are currently working on elucidating the reaction mechanism as well as improving our catalyst and reaction conditions," they continue. "Since we have already identified that the O-iminohydroxylamine is one of the reaction intermediates, we hope that we can report other new reactions that can lead to the rapid and efficient synthesis of useful compounds."