Cancer stem cells’ role in tumor growth revealed
Advertisement
Researchers at Okayama University have successfully created a mouse model suitable for studying the behaviour of cancer stem cells (CSCs) in tumors. Their initial insights clarify the role taken by CSCs in promoting tumor vasculature in the early stages of tumor growth.
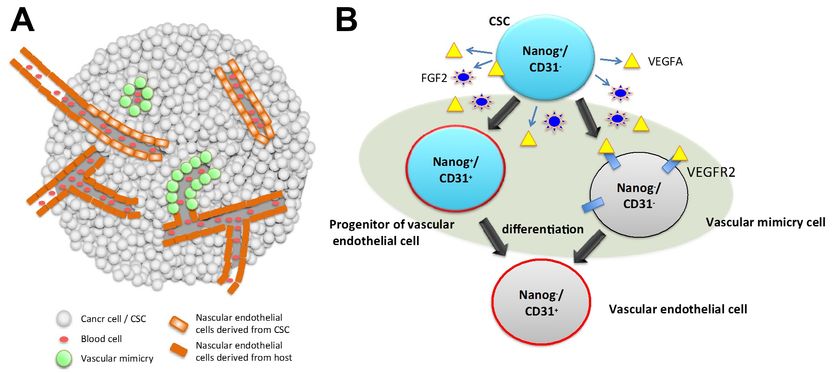
Components of vascular system in a tumor tissue (A) and the paths of differentiation from CSC to vascular endothelial cell (B). A, vascular system in a tumor tissue is composed of the cells derived from CSC connecting with the endothelial cells from the host. B, a CSC is pluripotent to differentiate into mature endothelial cells expressing CD31, endothelial cell marker, by way of heterogeneous intermediate stages, which are Nanog+ / CD31+ (a progenitor of endothelial cell) and Nanog- / CD31- (a vascular mimicry cell), where Nanog is considered as stemness marker.
Okayama University
In order to grow beyond a certain size, tumor cells trigger angiogenesis – a process by which new blood vessels form existing vessel structures. The tumor cells activate multiple mechanisms to develop blood vasculature, which then provides the tumor with the oxygen and nutrients it needs to grow.
While the basic processes surrounding tumor growth is reasonably well-understood, there is growing evidence to suggest that the formation of the vascular network is not restricted to angiogenesis. In fact, cancer stem cells (or CSCs) - cells within the tumor that can self-renew and differentiate into different cell types - appear to contribute to a phenomenon called ‘vasculogenic mimicry’; the formation of vascular-like channels without usual input from endothelial cells (common tissue cells) and growth factors.
Now, Professor Masaharu Seno and co-workers at Okayama University, together with scientists across Japan, China and the US, have generated a mouse model for assessing the role of CSCs in cancerous tumor development. Their results give considerable insights into the process of vasculogenic mimicry and may inform future therapeutic strategies.
The team had previously established their mouse model, miPSC-LLCcm, using mouse-induced pluripotent stem cells. They introduced the DsRed gene into the model which emits red-colored fluorescence and allowed the team to track CSCs during tumor growth. They found that angiogenic growth factors were predominantly expressed in one population of CSCs. This CSC group also worked to recruit endothelial cells from the host and promote the maturation of endothelial cells differentiated from the CSCs themselves.
Seno’s team then discovered that the remaining undifferentiated miPSC-LLCcm cells were directly involved in the formation of vasculogenic mimicry. These results indicate that tumor vasculature is a vital part of tumor growth, and highlights why therapies that solely target angiogenesis are not working as well as expected. As Seno states in their paper published in The American Journal of Cancer Research, “miPSC-LLCcm could be an appropriate model to understand entire tumor vascularization and to develop novel drugs and therapeutic strategies.”
Tumor growth
Tumors cannot grow very large without help from their host body. To survive and grow, tumor cells initiate the process of angiogenesis – creating new blood vessels from the existing tumor vessel structures. Inflammatory proteins and angiogenic factors are released by the tumor cells, which activates endothelial cells (common tissue cells found all over the body) and triggers degradation at the base of the tumor. This allows the endothelial cells to invade the surrounding membrane, multiply and migrate to form new blood vessels.
Despite this knowledge of growth influenced by angiogenesis, cancer therapies targeting angiogenesis alone have not been particularly successful. Seno and his team at Okayama University sought to clarify the role of other cancerous cells within the tumor – self-renewing cancer stem cells (CSCs) – to see if they also contribute to tumor vascularization.
The team’s results suggest CSCs are heavily involved in vascularization, both through recruiting endothelial cells from outside the tumor and differentiating into endothelial cells themselves. Further, the undifferentiated CSCs go on to mimic the formation of blood vessels, creating new vessel-like structures without the aid of other proteins and growth factors.
Implications of the current study
Seno’s team believe their results suggest further investigations are needed into CSC behaviour in tumor cells, and that their mouse model (miPSC-LLCcm) will provide scientists with the means to conduct these studies. They hope that their model will inform future targeted therapies for cancer.
Original publication
Prieto-Vila M, Yan T, Calle AS, Nair N, Hurley L, Kasai T, Kakuta H, Masuda J, Murakami H, Mizutani A, Seno M.; "iPSC-derived cancer stem cells provide a model of tumor vasculature"; Am J Cancer Res.; 2016