Lanthio Pharma expands management team
The Dutch biopharmaceutical company Lanthio Pharma B.V., a wholly owned subsidiary of MorphoSys AG, announced the appointment of Axel Mescheder, M.D. as Chief Medical Officer (CMO).
Dr. Heinz Schwer, Chief Executive Officer of Lanthio Pharma, commented: "We are very pleased to welcome Axel Mescheder at Lanthio Pharma where he will take a key role in the Company's management. We got to know him as an excellent clinical physician and experienced R&D manager and we are sure that he will further strengthen and complement our management team. Axel's main focus will be the development of Lanthio Pharma's lanthipeptide portfolio, in particular the preparation and subsequent execution of the clinical development of MOR107."
Dr. Mescheder has more than 20 years of experience in R&D management positions in the pharmaceutical and biotechnology industry. He has demonstrated the ability to successfully develop, implement and drive clinical development programs and has a track record of advancing a number of compounds through the various clinical phases that lead to product registration or label extensions, either in Europe or in the US. He joins Lanthio Pharma from Medpace GmbH, Munich/Germany, where he acted as Vice President Medical & Regulatory Affairs Europe. Prior to that, he held the positions of Chief Medical and Development Officer at TopoTarget A/S, Copenhagen/Denmark, and Medigene AG, Munich/Germany. From 2001 to 2003 he worked at MorphoSys AG as a Medical Director. Prior to that, Dr. Mescheder held various management positions in research and development at Genetics Institute/Wyeth-Ayerst Research (Pfizer), Centeon (CSL Behring) and Hoffmann-La Roche.
Other news from the department people

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
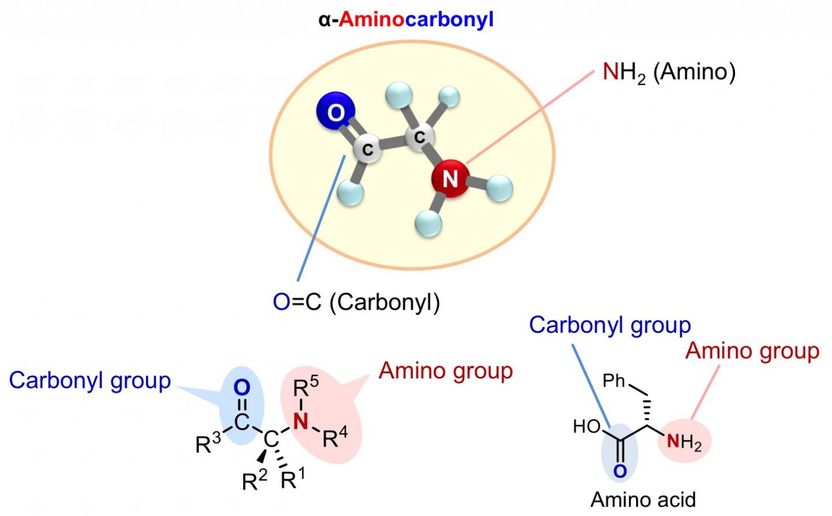
Rapid synthesis towards optically active alpha-aminocarbonyl therapeutics - New catalytic asymmetric reaction directly installs amines into carbonyl compounds
An inflammatory diet correlates with colorectal cancer risk - The risk of developing colorectal cancer for individuals that follow a pro-inflammatory diet is two times higher than usual