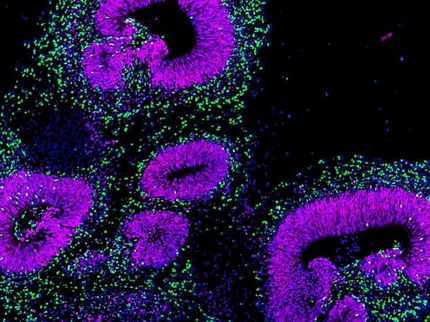
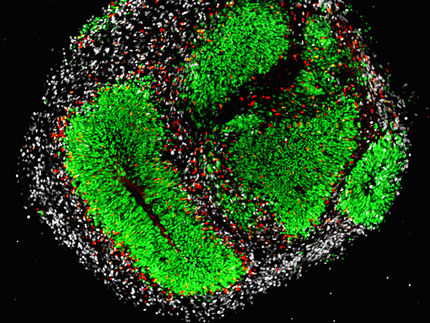
Genetic causes of small head size share common mechanism
microcephaly is a rare disorder that stunts brain development in utero, resulting in an abnormally small head. The zika virus is one environmental cause of this devastating condition, but genetic defects can cause microcephaly, too. A new Duke University study examining three genetic causes of microcephaly in mice suggests one common mechanism through which the disorder could arise.
The study offers a new window into early, critical stages of brain development, and may improve understanding of the diverse causes of microcephaly and other neurodevelopmental disorders, including autism.
“We’re excited about this study because, by stepping back and looking at the basic mechanistic routes to microcephaly, we hope to understand how Zika infection causes microcephaly,” said the study’s senior investigator Debra Silver, an assistant professor of molecular genetics and microbiology at the Duke University School of Medicine.
In the new study, Hanqian Mao, a graduate student in Silver’s lab, created three mouse models of microcephaly by cutting the levels of each of three genes -- Magoh, Rbm8a and Eif4a3 -- by half during a critical time in brain development. All three types of mice developed a smaller cerebral cortex, the part of the brain responsible for memory and thought.
Then, Mao screened for any changes in mRNA and protein levels that could also contribute to the underdeveloped brains. One change that stood out involved a protein called p53, which accumulated in each of the mutant brains. The group hypothesized that too much p53 could cause developing cells to die.
To test the involvement of p53 in microcephaly, Duke postdoctoral fellow John McMahon suppressed it in each of the three types of mice. By blocking p53 at a crucial point in development, the team was able to trigger the brains to partially or fully recover to normal size, suggesting that p53 or its signaling partners might be considered as new therapeutic targets for microcephaly.
“What we don’t know yet is exactly how our microcephaly-causing genes are regulating p53 and other changes in the brain, and that’s going to be the next big question,” Silver said.
The genes Magoh, Rbm8a and Eif4a3 are related to one another in that they bind together on specific spots on RNA and affect its processing to become protein. Although the triad is expressed in every cell of the body, it is more abundant in brain tissue.
“Our results suggest that the molecular complex is a master regulator of cortical development, because it’s regulating critical genes in stem cells, which must divide and then start making neurons,” said Silver, who is also a member of the Duke Institute for Brain Sciences.
“If you have problems at this early stage, you don’t get enough stem cells. And then the stem cells themselves can’t go on to make neurons. That’s where you get microcephaly,” Silver added.
Importantly, disruptions in the genes Rbm8a and Eif43 have already been linked to human cases of intellectual disability, and Rbm8a has been associated with microcephaly and autism in people.
“That’s another reason that identifying the downstream molecules of these genes is really important,” Silver said, adding that her team has some of the only mouse models in which it is possible explore those questions.
Next the group is interested in exploring whether microcephaly caused by Zika shares mechanisms with genetic cases of the disorder. They are now conducting preliminary studies in a developing Zika mouse model. “We’re well set up in the lab to ask how the Zika virus can shape brain development relevant to microcephaly,” Silver said.
Original publication
Hanqian Mao, John J. McMahon, Yi-Hsuan Tsai, Zefeng Wang and Debra L. Silver; "Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly"; PLOS Genetics; 2016
Original publication
Hanqian Mao, John J. McMahon, Yi-Hsuan Tsai, Zefeng Wang and Debra L. Silver; "Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly"; PLOS Genetics; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

Fungi-based meat alternatives to help save Earth’s forests - Green biotechnology needs to be fuelled by green energy