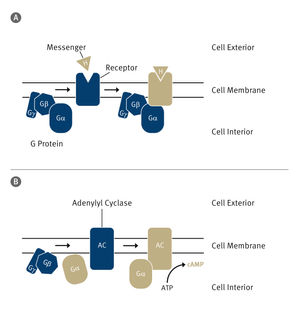
The first stage of the cascade
Switching mechanism of important signal protein signposts path to new medications
Advertisement
G proteins are molecular switches on the insides of cell membranes. They convey important signals to the inner workings of the cells. The associated receptors are targeted by all kinds of medications. Scientists at the Technical University of Munich (TUM) are now shedding light on precisely how the individual amino acids of the G protein move during the switching process. The discovered mechanism signposts new approaches to the design of new active agents.

Prof. Franz Hagn
Uli Benz / TUM
The human body is like a huge team project. Millions of cells, structured into tissue and organs, assume various tasks and support, coordinate between and regulate each other. For this collaboration to function the cells must exchange information readily. Specific proteins regulate this communication. As "messengers" they transmit the signals they receive from the outside world to the inner workings of the cells.
A messenger and its receptor in the cross-hairs of the pharmaceutical industry
So-called G proteins form an important class of these messenger proteins. They function as tiny molecular switches: When a signaling substance attaches to a G protein coupled receptor, the so-called alpha subunit of the G protein is "switched" on. It separates from the receptor and the other subunits and activates further proteins.
This is the first stage of a signaling cascade that culminates in the desired reaction. In a whole series of diseases, the regulation of this signal cascade is faulty, which explains why over 30 percent of all manufactured medications act on G protein coupled receptors. These include beta blockers, medications against high blood pressure and psychotropic drugs. Agents that act directly on G proteins are also conceivable.
More than just a snapshot – How exactly are the switches triggered?
Now a group of scientists led by Franz Hagn, Professor of Structural Membrane Biochemistry in the Department of Chemistry at TU Munich, has determined what precisely happens when the "switch" is flipped in an alpha subunit.
Using nuclear magnetic resonance spectroscopy, they resolved step by step how the individual amino acids move in the alpha subunit upon activation. "This insight may help manufacturers of medications create active agents precisely geared to the individual steps, something that has been difficult hitherto," says Franz Hagn.
G proteins investigated in natural state for first time
In their work, Hagn’s researchers were, for the first time, able to observe the movements of G protein alpha subunits in their natural environment, i.e. bound to a cell membrane. This is very difficult since membrane proteins are not soluble. But, it is a prerequisite for solution NMR spectroscopic investigations.
The scientists overcame this hurdle to investigating the G proteins by developing small membrane patches in which lipid binding proteins shielded the water-repelling edges. They then placed the G protein coupled receptors into theses phospholipid nanodiscs and examined the interactions with the soluble G protein.
The researchers determined that the receptor-bound form of the alpha subunit is very open when in the "off" position. When the activating guanosine triphosphate (GTP) binds to the protein, it snaps shut and the switch is activated. Now, the individual parts of the subunit rest together tightly. The complex is rigid and practically unalterable, which is essential for the activation of further signal proteins.
Prerequisites for future active agent development
The development of a medication that acts directly on the G protein is still a long way off. Nonetheless, the new insight indicates that the open form of the protein is more readily accessible by active agents than the rigid, closed form.
In follow-on research, the scientists headed by Hagn hope to also investigate the influence of G protein coupled receptors on the structure of the G protein, as well as the role of other G protein subunits in the switching process. Essential for this work will be the state-of-the-art technical facilities of the Bavarian NMR Center, which will be extended by a further high-field spectrometer on the Garching campus of TU Munich in the next two years.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!