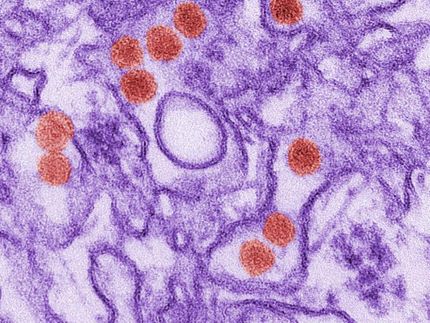
First diagnostic test for zika virus accepted by WHO
WHO has accepted the first Zika virus Diagnostic test eligible for procurement agencies and Member States. The RealStar® Zika Virus RT-PCR Kit 1.0 manufactured by altona Diagnostics was assessed under the Emergency Use Assessment and Listing Procedure (EUAL) opened to candidate in vitro diagnostics (IVDs) intended for Zika virus diagnosis in February 2016.
A public report with additional details about the WHO Emergency Use Assessment and Listing for RealStar® Zika Virus RT-PCR Kit 1.0. can be obtained via the link on the right side.
The EUAL procedure is an emergency quality assessment mechanism established by WHO during the 2014 Ebola Virus Disease outbreak. It was developed to expedite the availability of IVDs needed in public health emergency situations, based on a minimum set of available quality, safety, and performance data and an agreed plan for further evaluation.
Most read news
Organizations
Related link
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Leon_Kamin
LDC to Collaborate with Johnson & Johnson Innovation on Acceleration of Academic Drug Discovery Initiatives
Moth larvae spit boosts yield of Colombian spud
SciQuest Announces the Release of Enterprise Reagent Manager Version 4.4 - Enhanced reporting and purchasing gateway functionality designed to increase researcher productivity while optimizing environmental health and safety compliance
ARTES Biotechnology, instrAction and Q-Biologicals Join Forces for Veterinary Vaccine Development
ThromboGenics and BioInvent Announce Positive Topline Results from Phase II VTE Prophylaxis Study with Anti-Factor VIII (TB-402)
Study shows that mutations in 1 gene cause many cancers
Chondromalacia_patellae

Nanostructures with living cells
Category:English_marine_biologists