Neural stem cells control their own fate
To date, it has been assumed that the differentiation of stem cells depends on the environment they are embedded in. A research group at the University of Basel now describes for the first time a mechanism by which hippocampal neural stem cells regulate their own cell fate via the protein Drosha.
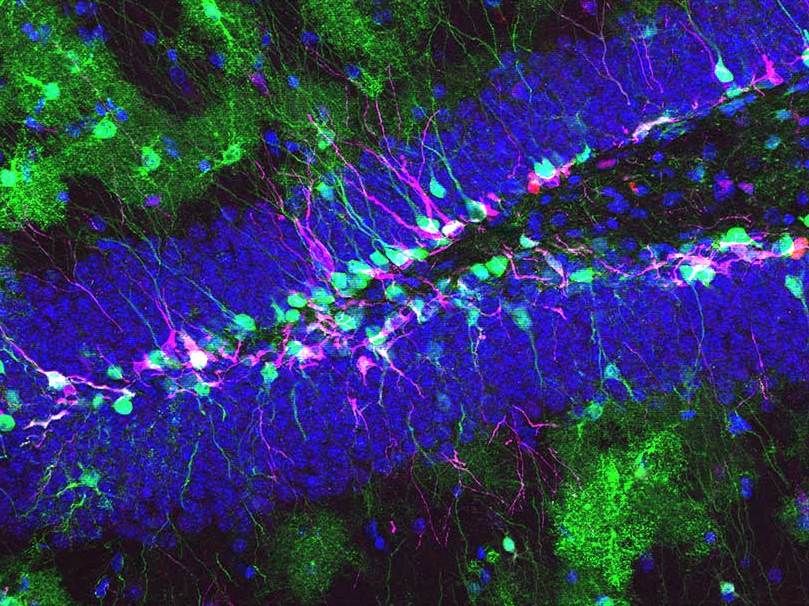
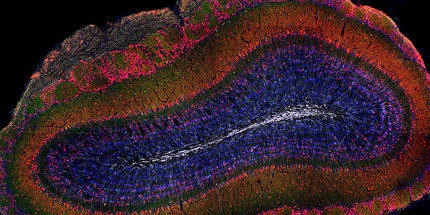
Neural stem cells in the adult mouse hippocampus. Gre the stem cells and their progeny express protein. Magenta: the hippocampal stem cells generate newborn neurons. Blue: mature granule neurons.
University of Basel, Department of Biomedicine
Stem cells are undifferentiated cells that have the potential to differentiate into many cell types. However, the cell types that somatic stem cells produce are usually restricted to those of the organ in which they sit. The current view proposes that stem cell differentiation is controlled by their local environment, the so-called niche. Thus, stem cells receive and interpret specific factors present in their niche that guide their differentiation into specific and restricted cell types.
In the adult brain, the hippocampus is responsible for specific forms of memory – a brain region that is also affected in diseases such as dementia, depression and epilepsy. The functions of the hippocampus are based on different cell types, some of which are generated throughout life by neural stem cells. Neural stem cells are generally accepted to produce three different cell types: neurons, astrocytes and oligodendrocytes. However, the adult hippocampus does not produce oligodendrocytes – the reason for this was so far not known.
Intrinsic cell mechanism
Researchers from the Department of Biomedicine at the University of Basel have now found that the fate of adult hippocampal stem cells is not only controlled by their local niche, but also by a cell-intrinsic mechanism. Their study describes the central role of the enzyme Drosha in this mechanism. Drosha degrades the messenger RNA for NFIB in the adult hippocampal stem cells and prevents the expression of this transcription factor which is necessary for the differentiation of oligodendrocytes and thus blocks their development and therefore biases differentiation towards neurons.
The team lead by Prof. Verdon Taylor was able to demonstrate for the first time a cell-intrinsic mechanism regulating stem cell fate. «Our research results about the function of Drosha challenge the way we used to think about how stem cell fate is controlled», says cell biologist Taylor. His research group now wants to study if and how stem cells are able to modulate the activity of Drosha in order to satisfy demand.
Original publication
Chiara Rolando, Andrea Erni, Alice Grison, Robert Beattie, Anna Engler, Paul J. Gokhale, Marta Milo,Thomas Wegleiter, Sebastian Jessberger, and Verdon Taylor; "Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB"; Cell Stem Cell; 2016
Most read news
Original publication
Chiara Rolando, Andrea Erni, Alice Grison, Robert Beattie, Anna Engler, Paul J. Gokhale, Marta Milo,Thomas Wegleiter, Sebastian Jessberger, and Verdon Taylor; "Multipotency of Adult Hippocampal NSCs In Vivo Is Restricted by Drosha/NFIB"; Cell Stem Cell; 2016
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Sacrum
Scurvy
Agilent Technologies to acquire Advanced Analytical Technologies, Inc.
Fairy_ring