Built-in Protective Mechanism against Inflammations: Kiel scientists investigate immune cells
The protein Interleukin-6 (IL-6) can take on different functions in cells, depending on how it activates the cells. If it activates cells via the classical signalling path, it helps with the regeneration of tissue, and is indispensable for fighting bacterial infections. However, if it activates cells via so-called ‘trans-signalling’, the protein propels inflammations. In the Journal of Biological Chemistry, scientists at Kiel University have now shown that human immune cells have a built-in protective mechanism which prevents them being activated via trans-signalling.
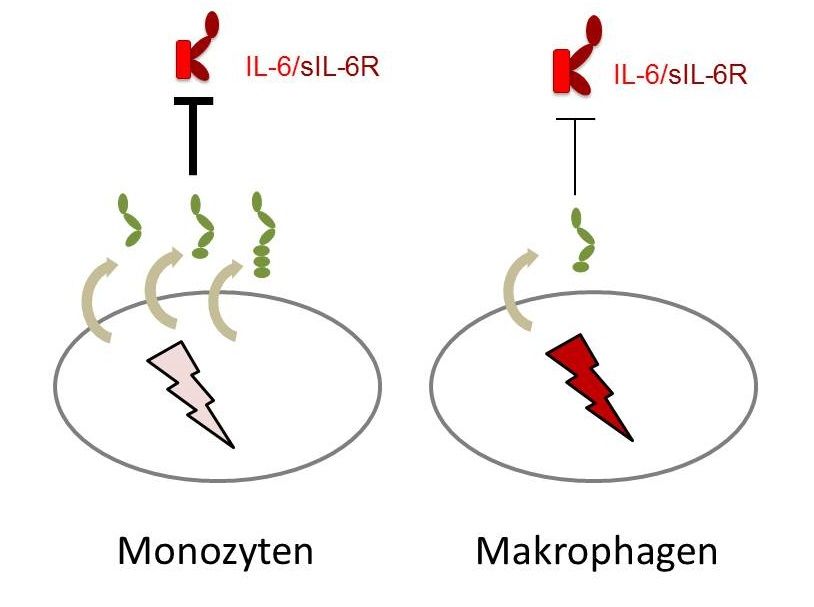
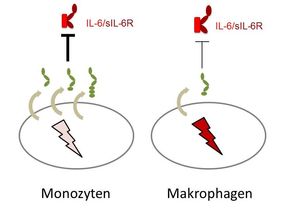
Monocytes, which are leukocytes, or white blood cells, excrete a lot of soluble gp130 in its longest form, and are thereby very well protected – but they lose this ability almost completely when they differentiate into macrophages.
Dr. Christoph Garbers
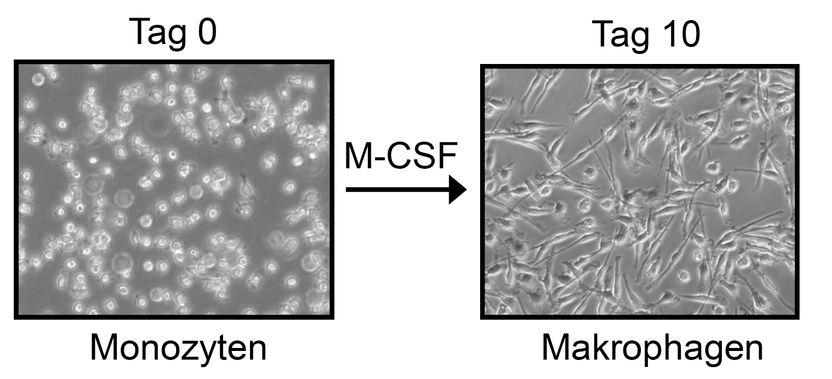
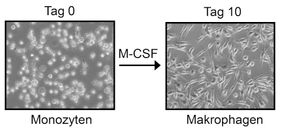
Microscopic recordings show monocytes (left), which scientists differentiated over ten days into macrophages by adding cytosin M-CSF.
Dr. Christoph Garbers
The protein Interleukin-6 (IL-6) unleashes its different effects by binding with Interleukin-6 receptors (IL-6R). These receptors exist in two versions: membrane-bound and soluble. The membrane-bound forms of the receptor are only found in very few cells in the human body. Through ‘classical signalling’, the regenerative properties of IL-6 are activated. In contrast, the soluble forms of the receptor can activate practically all the cells in the body unhindered, via the ‘trans-signalling’. “It is assumed that this signalling path, in particular, is responsible for triggering the inflammation-boosting activities of IL-6. Therefore, specifically blocking this 'trans-signalling' represents a potential therapeutic option,” said Dr. Christoph Garbers from the Institute of Biochemistry at Kiel University. Blocking this signalling path significantly improves the symptoms of many inflammatory diseases, and is used for treating rheumatoid arthritis, for example. To date, only one antibody has been approved for use which blocks the IL-6R, and thereby stops the activity of the protein.
Together with colleagues from Copenhagen and Hamburg, the Kiel researchers were now able to show that immune cells also have a built-in protective mechanism, to protect themselves from uncontrolled activation. For this purpose, they secrete soluble forms of the signal transducer gp130, which are able to bind with the complex comprising the protein IL-6 and the receptor sIL-6R, and thereby neutralise its activating effect.
It was already known in scientific circles that there are three forms of the soluble gp130 with different lengths. “However, no-one had previously investigated which cells can secrete which forms and, more importantly, why there are forms of different lengths in the first place,” said Garbers. The length of the gp130 forms influences the stability and effectiveness of the receptor: longer forms can block IL-6 trans-signalling more effectively than shorter ones. “We think that the shorter receptors are used for fine-tuning. As such, the cell has different adjustment mechanisms to defend itself against uncontrolled activation.” Whether or not this blockage can also be externally controlled is one of the topics that the researchers want to investigate next. “If we could stimulate cells to excrete much more of the long forms of gp130, this could be used in the treatment of inflammatory diseases.”
The research team discovered even more about the gp130 receptor: not only can it work differently; it is differentially expressed in a cell-type specific manner. “Interestingly, different immune cells display a different pattern of expression of the three soluble gp130 forms. This means that the cells have differing abilities to protect themselves against IL-6 trans-signalling,” said Garbers. “It is especially conspicuous that monocytes, which are leukocytes, or white blood cells, excrete a lot of soluble gp130 in its longest form, and are thereby very well protected – but they lose this ability completely when they differentiate into macrophages.”
What has not yet been investigated is how the gp130 pattern changes during illnesses. “Next, we would like to see whether changes occur during inflammatory diseases. If, for example, we find that more shorter forms are excreted, it would explain why the protein IL-6 has such pro-inflammatory effects,” said Garbers, looking ahead.



















































