An errant editing enzyme promotes tumor suppressor loss and leukemia propagation
Researchers at University of California San Diego School of Medicine report that detection of "copy editing" by a stem cell enzyme called ADAR1, which is active in more than 20 tumor types, may provide a kind of molecular radar for early detection of malignancies and represent a new therapeutic target for preventing cancer cell resistance to chemotherapy and radiation.
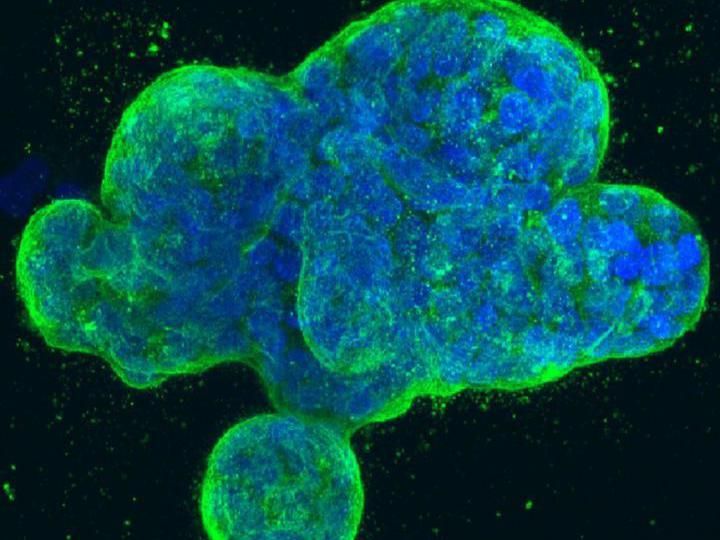
This is a three-dimensional culture of human breast cancer cells, with DNA stained blue and a protein in the cell surface membrane stained green.
NIH
Adenosine deaminases are a family of three enzymes encoded by the ADAR genes, which stand for adenosine deaminase acting on RNA. They regulate gene expression by modifying nucleotides within double stranded RNA molecules, serving as fundamental editors in the development of new stem cells.
The enzyme, however, is also activated in cancers as diverse as liver, breast and leukemia. A research team led by senior author Catriona Jamieson, MD, PhD, deputy director of the Sanford Stem Cell Clinical Center and deputy director of the UC San Diego Moores Cancer Center, found that the normal functions of the ADAR1 enzyme are hijacked by pre-malignant cells, leading to a cascade of molecular consequences that promote malignant transformation, dormant cancer stem cell generation and resistance to treatment.
"We were able to illuminate the abilities of ADAR1 to 'hyper-mutate' tumor suppressor RNAs in leukemia and, at the same time, edit the microRNA aimed at targeting the tumor suppressor RNA. This enzyme turns on cancer resistance via a domino effect on RNA instead of DNA," said first author Qingfei Jiang, PhD, assistant project scientist in Jamieson's lab.
Jamieson characterized RNA editing as tweaking basic genetic blueprints, not fundamentally rewriting them. Nonetheless, the results might be dramatic. "One result of detection of malignant RNA editing could be exposing dormant cancer stem cells that often escape therapies that target dividing cells, which leads to therapeutic resistance and disease relapse, and also highlight ADAR as a potentially tractable target for cancer stem cell elimination," said Jamieson.
Original publication
Qingfei Jiang, Jane Isquith, Maria Anna Zipeto, Raymond H. Diep, Jessica Pham, Nathan Delos Santos, Eduardo Reynoso, Julisia Chau, Heather Leu, Elisa Lazzari, Etienne Melese, Wenxue Ma, Rongxin Fang, Mark Minden, Sheldon Morris, Bing Ren, Gabriel Pineda, Frida Holm, Catriona Jamieson; "Hyper-Editing of Cell-Cycle Regulatory and Tumor Suppressor RNA Promotes Malignant Progenitor Propagation"; Cancer Cell; 2019
Most read news
Original publication
Qingfei Jiang, Jane Isquith, Maria Anna Zipeto, Raymond H. Diep, Jessica Pham, Nathan Delos Santos, Eduardo Reynoso, Julisia Chau, Heather Leu, Elisa Lazzari, Etienne Melese, Wenxue Ma, Rongxin Fang, Mark Minden, Sheldon Morris, Bing Ren, Gabriel Pineda, Frida Holm, Catriona Jamieson; "Hyper-Editing of Cell-Cycle Regulatory and Tumor Suppressor RNA Promotes Malignant Progenitor Propagation"; Cancer Cell; 2019
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.