Cell Death: How a Protein Drives Immune Cells to Suicide
For some pathogens, attack is the best form of defense – they enter immune cells of the human body. However, if they are detected in their hidden niche, the infected cell kills itself to re-expose the pathogens. In the EMBO Journal a research group at the University of Basel’s Biozentrum has reported that a protein called gasdermin forms permeable pores in the cell membrane and thus triggers the suicide of the immune cell.
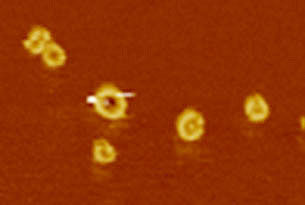
Atomic force microscopy image of the pore.
University of Basel, Biozentrum
The best hiding place often lies behind enemy lines, as many bacteria such as the pathogens responsible for tuberculosis or typhoid have realized. They invade immune cells and can survive there, well hidden, for some time. To eliminate such invaders, the host macrophages can initiate a suicide program. Together with researchers at the Novartis Institute for Biomedical Research and ETH Zurich, the team led by Prof. Sebastian Hiller from the Biozentrum at the University of Basel has shown for the first time that a “death protein” perforates the cell membrane, resulting in macrophage bursting open. The re-exposed pathogens can then again be fought by the immune system.
Gasdermin D: Executioner in the cell
Tiny cell components of invading pathogens are recognized by receptors inside macrophages. These, in turn, activate a signaling cascade, which triggers an inflammatory response and initiates pyroptosis – a specific form of programmed cell death. “Some studies have already demonstrated that the protein gasdermin D plays a central role in pyroptosis,” explains Prof. Petr Broz, one of the main authors of the study. “We have now discovered how gasdermin drives immune cells to suicide and using cryo-electron and atomic force microscopy we could visualize the pores in the cell membrane.”
The protein gasdermin D is at the end of a long signaling pathway. Intracellular receptors recognizing foreign bacterial components induce the assembly of the inflammasome. This protein complex, in turn, activates enzymes that generate active gasdermin fragments by proteolytic cleavage. “In the macrophages, gasdermin D is the executioner, which carries out the death sentence,” says Hiller, clarifying the role of the protein. “The cleaved gasdermin D fragments rapidly target the cell membrane of macrophages and permeabilize it by forming a pore. The porous membrane leads to cell swelling and bursting.”
Gasdermins cooperate in cell suicide
With gasdermin D, the researchers have not only identified the protein that deals a deathblow to immune cells, but they could also visualize pore formation using high-resolution microscopy techniques. As it turns out, following the cleavage of gasdermin D only one of the two fragments is required for the seamless integration into the cell membrane. So far, only little is known about the gasdermin family of proteins, which along with gasdermin D includes five other members. In the future, Hiller’s team aims to investigate the structure and function of several gasdermins to determine whether and how they cooperate in pore formation and to identify the physiological context in which these proteins induce pyroptotic cell death.
Original publication
Lorenzo Sborgi, Sebastian Rühl, Estefania Mulvihill, Joka Pipercevic, Rosalie Heilig, Henning Stahlberg, Christopher J. Farady, Daniel J. Müller, Petr Broz and Sebastian Hiller; "GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death"; EMBO Journal; published online 14 July 2016