Preclinical study outlines cardiovascular side effects of breast cancer drug
A receptor protein that is the target of the breast cancer drug trastuzumab (Herceptin) is needed for proper heart blood-vessel development, reported researchers from the Perelman School of Medicine at the University of Pennsylvania. These discoveries have implications for better understanding the cardiovascular side effects of trastuzumab commonly used for cancer and provide an example of integration at the molecular level of pathways involved in tissue growth and blood-vessel patterning.
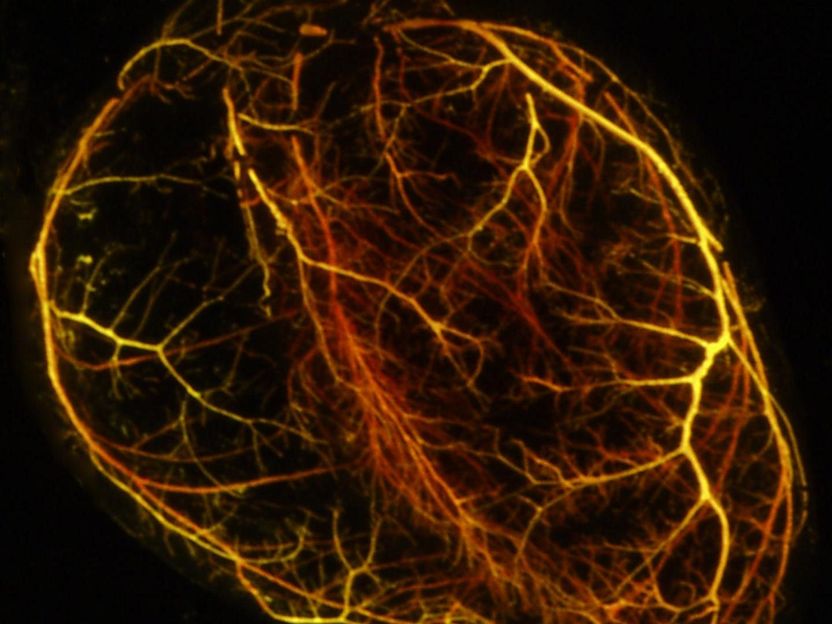
This is a visualization of the coronary vasculature patterning of an adult mouse heart
Haig Aghajanian, Ph.D., Perelman School of Medicine at the University of Pennsylvania and Young Kuk Cho
First author Haig Aghajanian, PhD, a postdoctoral fellow in the lab of senior author Jon Epstein, MD, a professor of Cell and Developmental Biology and executive vice dean and chief scientific officer for Penn Medicine, showed that ErbB2, a growth factor receptor, is unexpectedly expressed by vascular endothelial cells. Here it partners with neuropilin 1 to form a receptor for the vascular guidance molecule semaphorin 3d. Guidance molecules work by instructing the direction in which cells migrate, such as when new blood vessels are forming.
"ErbB2 is a well known target in cancer therapy, but not in vascular biology," Aghajanian said. "Our work identifies a new role for this important protein in blood vessel development and gives us a possible explanation for some of the cardiovascular side effects associated with anti-ErbB2 therapies."
They found that the loss of semaphorin 3d leads to improper connections of the coronary veins forming within the developing heart. They found a similar effect when ErbB2 was lost from developing heart-vessel-lining cells, providing a genetic link between these pathways.
The team was surprised when ErbB2 came up in their search for molecular partners for semaphorin 3d because semaphorin guidance molecules are not typically known to work through ErbB receptors.
"We questioned ErbB2's role at first," Epstein said. (ErbB2 had not previously been shown to even be in blood vessels.) "But now these findings give us a possible explanation for why there can be serious cardiovascular side effects, including heart failure, in patients receiving anti-ErbB2 therapies such as Herceptin."
Aghajanian and Epstein are using this knowledge to refine the hypothesis that targeting ErbB2 may also affect heart blood vessels leading to unwanted side effects. A better understanding of why the deleterious side effects are produced may lead to better ways to monitor and detect these unwanted effects before they cause symptoms.
"This work adds to an array of signals that affect how vessels grow and offers more targets to develop that promote or block the growth of blood vessels," Epstein said. These new targets could have implications for stopping the growth of certain tumors, abating ischemia, and alleviating diabetic retinitis.























































