Merck and Pfizer Initiate Phase III Trial to Evaluate Avelumab as First-line Treatment for Ovarian Cancer
Merck and Pfizer announced the initiation of a Phase III study, JAVELIN Ovarian 100, to evaluate the efficacy and safety of avelumab in combination with, and/or as follow-on (maintenance) treatment to, platinum-based chemotherapy in patients with locally advanced or metastatic disease (Stage III or Stage IV) with previously untreated epithelial ovarian cancer. JAVELIN Ovarian 100 is the first Phase III study evaluating the addition of an immune checkpoint inhibitor to standard-of-care in first-line treatment for this aggressive disease.
“In an early ongoing study, avelumab showed encouraging tumor response rates in patients with recurrent or refractory ovarian cancer,” said Alise Reicin, M.D., Head of Global Clinical Development in the biopharma business of Merck. “Historically, ovarian cancer presents as an advanced disease with poor survival rates. The hope is that avelumab can change the natural history of the disease and potentially take the survival rate beyond the current five year estimate.”
JAVELIN Ovarian 100 is an open-label, international, multi-center, randomized (1:1:1) Phase III trial in treatment-naïve patients with locally advanced or metastatic ovarian cancer (Stage III or Stage IV). This study is designed to evaluate the potential superiority of two first-line therapies with avelumab and platinum-based chemotherapy versus platinum-based chemotherapy alone, as assessed by progression-free survival. The study will enroll approximately 950 patients, who will receive concurrent avelumab and chemotherapy, avelumab following chemotherapy, or chemotherapy alone.
“Patients with ovarian cancer need additional treatment options. We believe there could be synergistic activity in the combination of avelumab and established treatments such as platinum-based chemotherapy,” said Chris Boshoff, M.D., Ph.D., Head of Early Development, Translational and Immuno-Oncology, Oncology in Pfizer Global Product Development. “With two studies now underway of avelumab in ovarian cancer, we look forward to receiving the results from these trials and continuing to break ground in this hard-to-treat cancer.”
The alliance aims to build a strong foundation in ovarian cancer. In December 2015, Merck and Pfizer announced the initiation of an international Phase III study of avelumab as a treatment for platinum-resistant/refractory ovarian cancer. As of May 2016, the complete JAVELIN clinical development program for avelumab includes approximately 2,200 patients enrolled, being treated across more than 15 tumor types.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
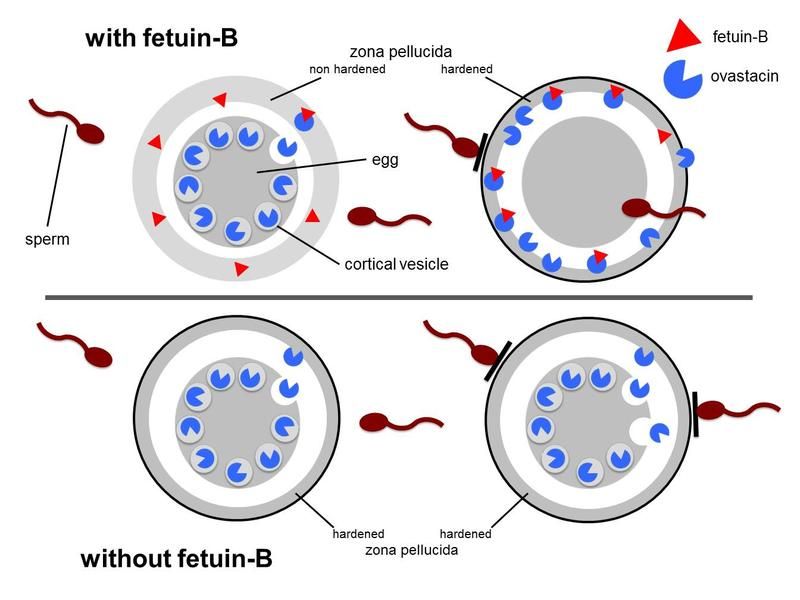
Important fertility mechanism discovered - Plasma protein fetuin-B regulates hardening of the zona pellucida and thus ensures the fertilization capacity of the ovum
Orthobiologics Are the Future for Bone Healing - Innovations in biomaterials, graft design and other technologies have pushed these products to the forefront of the industry, says Frost & Sullivan
Genome Alberta - Kanada, Canada






















































