Study shows how genes affect immunity in response to pathogens
Advertisement
A study that is first in its kind has looked at how far genetic factors control the immune cell response to pathogens in healthy individuals. A team investigated the response of immune cells from 200 healthy volunteers when stimulated with a comprehensive list of pathogens ex vivo (outside the human body), and has correlated these responses with 4 million genetic variants (SNPs). The study was performed by scientists from University Medical Centre Groningen, Radboud University Medical Centre (both in the Netherlands) and Harvard Medical School (Boston, USA). The paper appeared on 4th of July 2016.
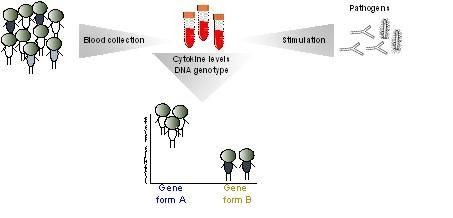
This is a schematic overview of the study into the response of immune cells to stimulation with varied pathogens.
Yant Li, Vinod Kumar, UMCG
We all encounter pathogens on a daily basis, but we don't all defend ourselves against bacteria or fungi, for example, in the same way. Some people experience mild symptoms, others may become violently ill or even die. 'We wanted to discover how much individual genetic differences determine this variable response', said Dr. Vinod Kumar, assistant professor of functional genomics and infectious diseases at University Medical Centre Groningen (UMCG) and one of the senior authors of the paper.
The study focused on the role of cytokines, small peptides used by immune cells as signals to guide their response to an infectious agent. Blood samples were obtained from 200 participants in the Human Functional Genomics Project, which was initiated by Professors Mihai Netea and Leo Joosten (Radboud UMC) and Prof. Cisca Wijmenga (UMCG). Immune cells were isolated from the blood and stimulated in the laboratory with ten different bacterial and fungal pathogens. The responses of eight different cytokines were measured after 24 hours and/or 7 days. Further quality filtering resulted in 62 different combinations.
Varied response
'We observed large differences in cytokine production between individuals', explained Kumar. 'Their responses were also specific to the different pathogens'. This suggests that cytokines contribute to the varied responses to pathogens, and that each infection triggers a specific cytokine response pathway. Previous studies on unstimulated immune cells had shown little variation between individuals.
Dr. Yang Li, another author on the study, explained: 'The key to our results is that we performed a large-scale study, using many pathogens and measuring different cytokines'. The next step was to investigate if the responses were under genetic control. In a subset of participants, they tested 4 million single nucleotide polymorphisms (SNPs).
This pinpointed six genomic regions that influence cytokine responses, suggesting that cytokine production is at least partly genetically determined. One of the strongest examples is a SNP that affects the expression of the GOLM1 gene, which is known to express strongly in response to viral infections. In this case, there was a strong correlation with the response to the fungus Candida albicans, which is responsible for thousands of deaths each year.
Proof-of-concept
'When a particular variant of the SNP was present, the production of the cytokine interleukin-6 was reduced', said Kumar. The result was verified in a cohort of patients with candidemia, in which the fungus is present in the blood. The candidemia correlated strongly with the same SNP and with low levels of interleukin-6 in these patients, showing that the presence of the genetic variant results in an inability to clear the pathogen.
'This study is a real proof-of-concept', says Li. 'We found a lot of variation in cytokine production upon stimulation, and showed that an important part of this variation is explained by a genetic component'. This opens the way for more applied studies that move towards personalized medicine. It might well be possible to find genetic markers that will predict the risk of infection in individuals. An understanding of the genetic mechanisms underlying these different susceptibilities could lead to new therapeutic approaches.
'And it's not just for infections', said Kumar: 'Immune diseases, for example inflammatory bowel disease, appear to be caused by an over-responsive immune system. So this work means we can learn more about the way infections trigger immune diseases.'
Original publication
Yang Li, Marije Oosting, Patrick Deelen, Isis Ricaño-Ponce, Sanne Smeekens, Martin Jaeger, Vasiliki Matzaraki, Morris A Swertz, Ramnik J Xavier, Lude Franke, Cisca Wijmenga, Leo A B Joosten, Vinod Kumar & Mihai G Netea; "Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi"; Nature Medicine; 2016