E. coli: The ideal transport for next-gen vaccines?
Most people recoil at the thought of ingesting E. coli. But what if the headline-grabbing bacteria could be used to fight disease?
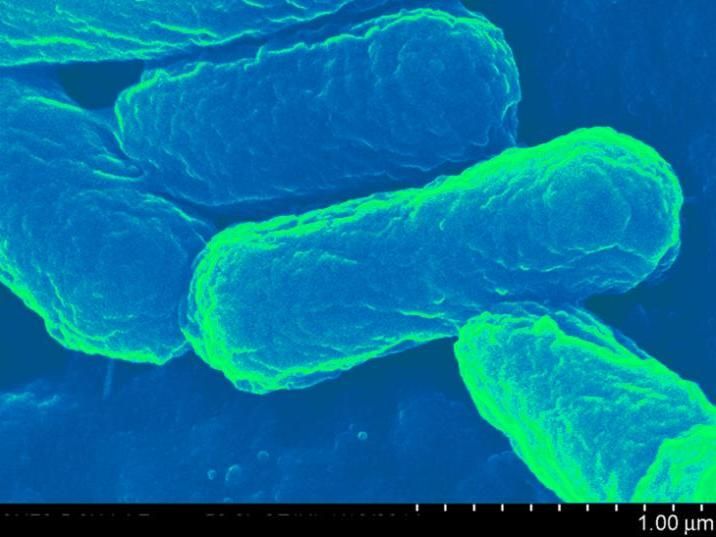
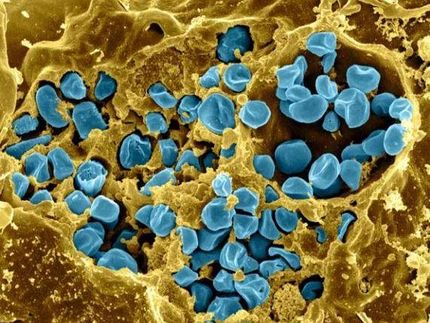
The core of the capsule is harmless E. coli. Around the bacteria, the researchers wrapped a synthetic polymer -- called poly (beta amino ester) - like a chain link fence. The positive-charged polymer, combined with the negative-charged bacteria cell wall, create a sort of hybrid capsule.
University at Buffalo
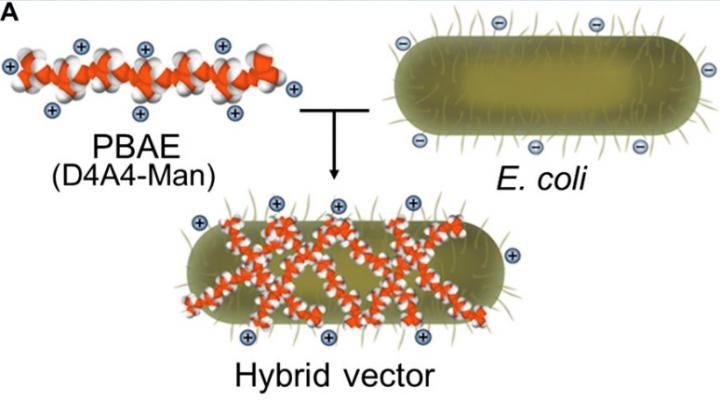
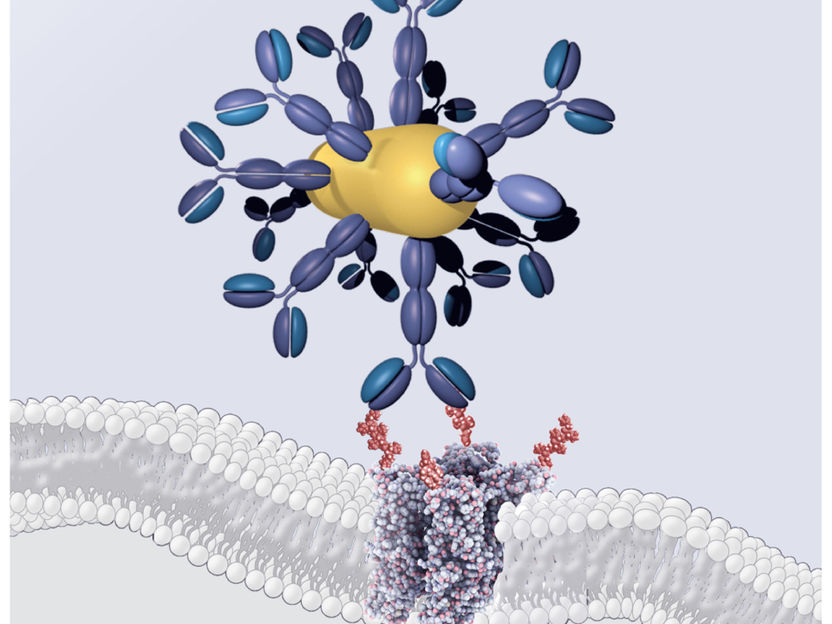
Here is a rendering of how the synthetic polymer -- called poly (beta amino ester) wraps around the E. coli-based core creating a sort of hybrid capsule.
University at Buffalo.
Researchers experimenting with harmless strains of E. coli - yes, the majority of E. coli are safe and important to healthy human digestion - are working toward that goal. Specifically, they have developed an E. coli-based transport capsule designed to help next-generation vaccines do a more efficient and effective job than today's immunizations.
The research, described in a study highlights the capsule's success fighting pneumococcal disease, an infection that can result in pneumonia, sepsis, ear infections and meningitis.
"It's a bit counterintuitive given what you here about E. coli, but there are many strains of the bacteria, most of which are perfectly normal in the body that have great potential to fight disease," said Blaine A. Pfeifer, PhD, associate professor of chemical and biological engineering in the University at Buffalo School of Engineering and Applied Sciences.
Pfeifer is the study's co-lead author along with his former student Charles H. Jones, PhD, who is leading efforts to commercialize the biotechnology as CEO and founder of Buffalo, New York-based startup Abcombi Biosciences.
The core of the capsule is harmless E. coli. Around the bacteria, the researchers wrapped a synthetic polymer - called poly (beta amino ester) - like a chain link fence. The positive-charged polymer, combined with the negative-charged bacteria cell wall, create a sort of hybrid capsule.
To test the capsule, the researchers then inserted a protein-based vaccine, also being commercialized by Abcombi, designed to fight pneumococcal disease. The results, when tested in mice, were impressive.
The capsule's hybrid design provided:
- Both passive and active targeting of specific immune cells called antigen-presenting cells that trigger an immune response.
- Natural and multicomponent adjuvant properties, which enhance the body's immune response.
- Dual intracellular delivery mechanisms to direct a particular immune response.
- Simultaneous production and delivery of the components (antigens) required for a vaccine.
- Strong vaccination protection capabilities against pneumococcal disease.
It's also relatively inexpensive to create and flexible in terms of use. For example, the capsule could be used as a delivery device for therapies that target cancer, viral-based infectious disease and other illnesses.
Original publication
Yi Li, Marie Beitelshees, Lei Fang, Andrew Hill, Mahmoud Kamal Ahmadi, Mingfu Chen, Bruce A. Davidson, Paul Knight III, Randall J. Smith, Jr, Stelios T. Andreadis, Anders P. Hakansson, Charles H. Jones, Blaine A. Pfeifer; "In situ pneumococcal vaccine production and delivery through a hybrid biological-biomaterial vector"; Science Advances; 2016