Iowa State researchers describe copper-induced misfolding of prion proteins
Iowa State University researchers have described with single-molecule precision how copper ions cause prion proteins to misfold and seed the misfolding and clumping of nearby prion proteins.
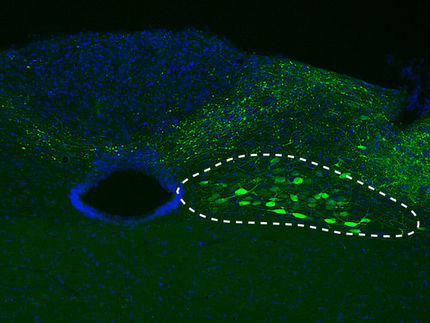
The researchers also found the copper-induced misfolding and clumping is associated with inflammation and damage to nerve cells in brain tissue from a mouse model.
Prions are abnormal, pathogenic agents that are transmissible and induce abnormal folding of a specific type of protein called prion proteins, according to the Centers for Disease Control and Prevention. Prion proteins are mostly found in the brain. The abnormal folding of prion proteins leads to brain damage and symptoms of neurodegenerative disease. A similar cycle of neuronal protein misfolding and clumping is observed in other neurodegenerative disorders, including Parkinson's and Alzheimer's diseases.
"Our study establishes a direct link, at the molecular level, between copper exposure and prion protein neurotoxicity," the researchers wrote in a summary of the paper.
The corresponding author is Sanjeevi Sivasankar, an Iowa State University associate professor of physics and astronomy; the first author is Chi-Fu Yen, an Iowa State doctoral student in electrical and computer engineering. Co-authors are Anumantha Kanthasamy, an Iowa State Clarence Hartley Covault Distinguished Professor in Veterinary Medicine, chair of biomedical sciences and director of the Iowa Center for Advanced Neurotoxicology; and Dilshan Harischandra, an Iowa State doctoral student in biomedical sciences.
Grants from the National Institute of Environmental Health Sciences at the National Institutes of Health supported the project, including one from the Virtual Consortium for Transdisciplinary Environmental Research.
Although this study determined that copper-induced misfolding and clumping of prion proteins is associated with the degeneration of nerve tissues, Sivasankar cautioned that the study does not directly address the infectivity of prion diseases.
"There are different strains of misfolded prion proteins and not all of them are pathogenic," Sivasankar said. "Although we do not show that the strains generated in our experiments are infectious, we do prove that copper ions trigger misfolding of prion proteins which causes toxicity in nerve cells."
The Sivasankar and Kanthasamy research groups plan to perform additional studies to determine if the copper-induced misfolding causes disease.
Integrating approaches Sivasankar also noted that a unique aspect of this project was the integration of biophysical and neurotoxicological research approaches. He said the combination has the potential to transform studies of the molecular basis for neurodegenerative diseases.
The biophysical approaches Sivasankar's team developed for this study include:
A fluorescence-based technique that identified misfolded prion proteins with single-molecule sensitivity and determined the role of metal ions in misfolding. The researchers used this technique to show that misfolding begins when copper ions bind to the unstructured tail of the prion protein. A single-molecule atomic force microscopy assay that measured the efficiency of prion protein clumping. The researchers used this technique to show that misfolded prion proteins stick together nearly 900 times more efficiently than properly folded proteins.
The Kanthasamy and Sivasankar research groups worked together on a real-time, quaking-induced conversion assay to demonstrate that misfolded prion proteins serve as seeds that trigger the misfolding and clumping of nearby prion proteins. Kanthasamy's research group also used its expertise in neurotoxicology to show the copper-induced, misfolded prion proteins damage nerve cells in slices of brain tissue from mice.
Taken together, the results identify the biophysical conditions and mechanisms for copper-induced prion protein misfolding, clumping and neurotoxicity, the researchers wrote.
"This was a very comprehensive study," Sivasankar said. "We took it from single molecules all the way to tissues."
And, although the study doesn't address the infectious nature of prion diseases, Kanthasamy said it is still important: "This study has major implications to our understanding the role of metals in protein misfolding diseases including prion, Alzheimer's and Parkinson's diseases."