Next generation of viral vectors, called AAV 3.0, for gene therapies and genome editing
The Perelman School of Medicine at the University of Pennsylvania has launched a new program, called AAV 3.0™, to create new viral vectors to find quicker and better treatments for an array of diseases. James M. Wilson, MD, PhD, a professor of Medicine and director of the Orphan Disease Center, will lead an interdisciplinary team of over 30 scientists to create this new technology platform with support provided by the University of Pennsylvania Health System.
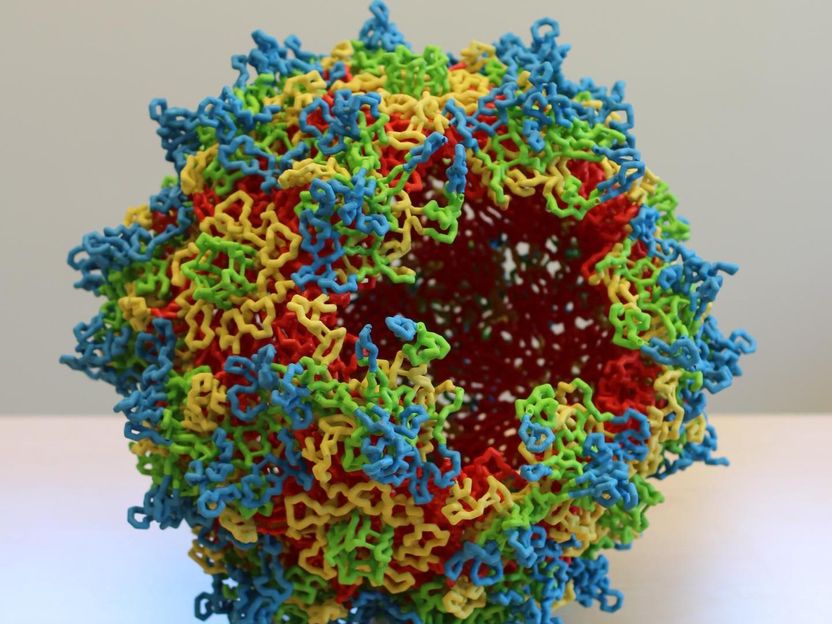
A 3-D model of adeno-associated virus (AAV) capsid structure: AAV capsid sixty subunits assemble to generate the icosahedral symmetric virus capsid. The viral asymmetric unit of the capsid is bounded by 5-fold, 3-fold, and 2-fold symmetry axes. The capsid external surface features are formed by the subunit hyper-variable loops.
Katherine Hinkel, University of Pennsylvania
The current wave of clinical applications of gene therapy is largely based on a new family of adeno-associated virus (AAV) vectors -- the most commonly used viral vectors today -- developed over 10 years ago by Wilson's laboratory at Penn. AAVs are used to ferry corrected genes and other small nucleic acids into cells. Translation of AAV gene therapy into the clinic has largely been in the treatment of disabling and lethal rare genetic diseases where the need is the greatest.
"I am optimistic that successful technologies will continue to emerge from our existing AAV vectors to help patients suffering from severe inherited diseases," Wilson said. "However, we believe it is possible to significantly improve the performance of future AAV vectors to expand their clinical utility for gene therapy and to help usher in therapeutic applications of genome editing."
Penn has been a catalyst for the scientific, clinical, and commercial development of AAV gene therapy through an extensive network of private and public collaborations. A dozen biopharmaceutical licensees including two companies Penn helped to form -- REGENXBIO and Dimension Therapeutics -- are currently using earlier AAVs involving over 26 diseases.
"With this newest program in AAV technology, Penn Medicine continues to build upon its groundbreaking work to facilitate the development of next-generation vectors and therapies, as well as the clinical translation of those efforts," said J. Larry Jameson, MD, PhD, Executive Vice President of the University of Pennsylvania for the Health System and Dean of the Perelman School of Medicine.
The University and Penn Medicine have long contributed to pioneering work in the clinical translation of cell and gene therapy. Examples include the treatment of patients with inherited blindness with gene therapy by Jean Bennett, MD, PhD, the F. M. Kirby professor of Ophthalmology and director of Penn's Center for Advanced Retinal & Ocular Therapeutics, and the use of chimeric antigen receptor (CAR) T cells to treat patients with leukemia by Carl June, MD, the Richard W. Vague Professor of Immunotherapy in the department of Pathology and Laboratory Medicine and director of Translational Research in the Abramson Cancer Center. In addition, the first gene therapy product approved in the Western hemisphere, used for the treatment of a rare genetic form of pancreatitis, uses a vector developed by Wilson.
"Our current successes in cell and gene therapy represent decades of work by our scientists and unwavering support by the institution," said Ralph W. Muller, CEO of the University of Pennsylvania Health System. "We stand prepared to do what is necessary to make sure these life-saving therapies are made available to our patients."
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.