Pfizer to acquire Anacor
Pfizer Inc. and Anacor Pharmaceuticals, Inc. announced that they have entered into a definitive merger agreement under which Pfizer will acquire Anacor for $99.25 per Anacor share, in cash, for a total transaction value, net of cash, of approximately $5.2 billion, which assumes the conversion of Anacor’s outstanding convertible notes. The Boards of Directors of both companies have unanimously approved the transaction. Anacor’s flagship asset, crisaborole, a differentiated non-steroidal topical PDE4 inhibitor with anti-inflammatory properties, is currently under review by the U.S. FDA for the treatment of mild-to-moderate atopic dermatitis, commonly referred to as eczema.
In both of its Phase 3 pivotal studies, crisaborole achieved statistically significant results on all primary and secondary endpoints and in March 2016, the FDA accepted for review Anacor’s New Drug Application seeking approval of crisaborole for the potential treatment of mild-to-moderate atopic dermatitis in children and adults. The Prescription Drug User Fee Act (PDUFA) goal date for the completion of the FDA’s review is January 7, 2017. If approved, Pfizer believes peak year sales for crisaborole have the potential to reach or exceed $2.0 billion.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Panaeolus_semiovatus_var._phalaenarum
Nicholas_A._Cummings_(Nick_Cummings)
Mane_(horse)
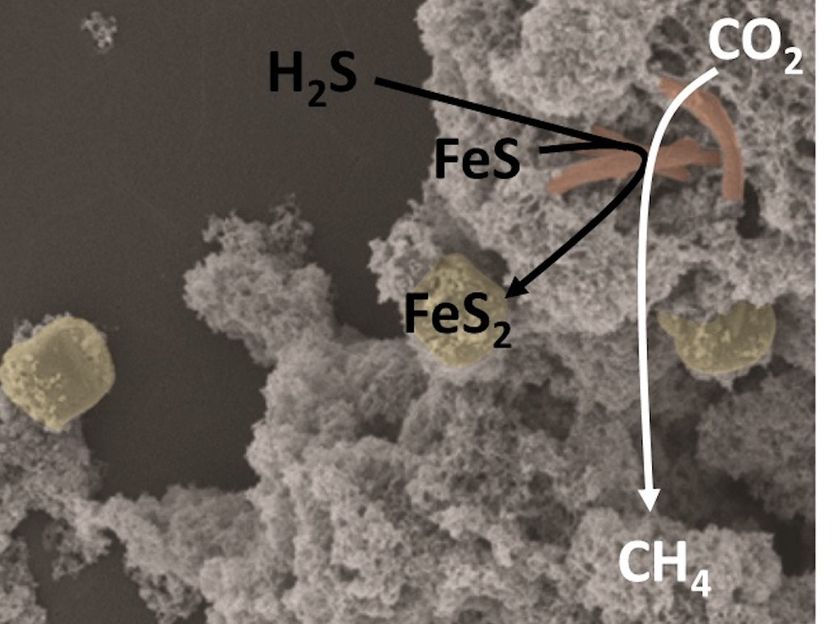
Discovery of a Primordial Metabolism in Microbes
Category:Molluscicides
Proteins may point to alcohol use test
Brain inflammation targeted in first drug discovery project from £3m Dementia Consortium
Centre_for_Infectious_Disease_Research_in_Zambia_(CIDRZ)
Tiotropium
First open genetic testing accreditation workshop proves a major success - Participants from across Europe welcome initiative