High-speed camera snaps biosensor's rapid reaction to light
X-ray study reveals ultrafast dynamics of photoactive yellow protein
Advertisement
Using a high-speed X-ray camera, an international team of scientist including researchers from DESY has revealed the ultrafast response of a biosensor to light. The study, published in the US journal Science, shows light-driven atomic motions lasting just 100 quadrillionths of a second (100 femtoseconds). The technique promises insights into the ultrafast dynamics of various light sensitive biomolecules responsible for important biological processes like photosynthesis or vision.
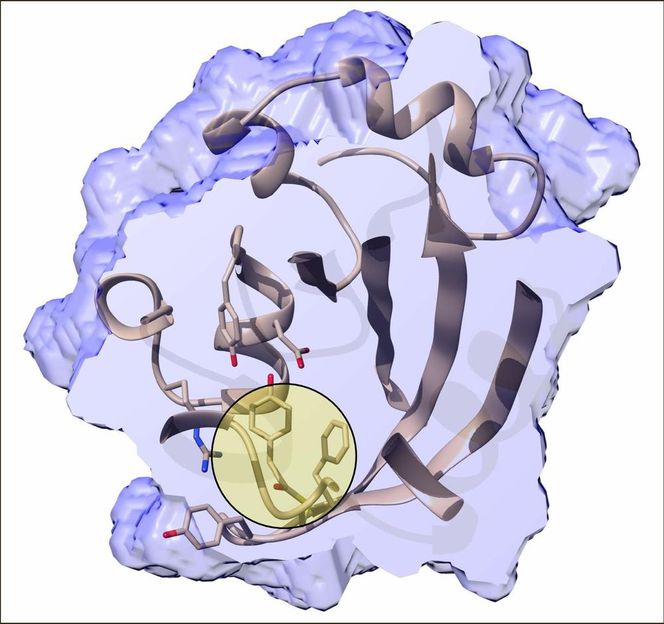
Inner structure of the photactive yellow protein 800 femtoseconds after the trans-to-cis isomerisation has been initiated by an ultrafast blue laser. The chromophore binding pocket is cut open and the chromophore itself is highlighted by the bulls eye.
Marius Schmidt/University of Wisconsin-Milwaukee
The team lead by Marius Schmidt from the University of Wisconsin, Milwaukee used the LCLS X-ray laser at SLAC National Accelerator Laboratory in the U.S. to look at the light-sensitive part of a protein called photoactive yellow protein, or PYP. It functions as an “eye” in purple bacteria, helping them sense blue light and stay away from light that is too energetic and potentially harmful.
For their investigation, the scientists sent a stream of tiny PYP crystals into a sample chamber. There, each crystal was struck by a flash of optical laser light and then, almost immediately after, an X-ray pulse was used to interrogate the protein’s structural response to the light at the atomic level. The structure is determined indirectly from the intricate pattern of X-ray light scattered from the crystal. By varying the time between the two pulses, scientists were able to see how the protein morphed over time. “By placing the various obtained molecular structures in order of the time delay between the optical and X-ray flashes we obtain a molecular movie of the reaction as it evolves from the first step at 100 femtoseconds to several thousand femtoseconds,” explained the first author of the paper, Kanupriya Pande, also from the University of Wisconsin and now at the Center for Free-Electron Laser Science CFEL at DESY.
“The absorption of light leaves PYP in an excited state from which it relaxes very quickly,” explained Schmidt, the study's principal investigator. “It does so by rearranging its atomic structure in what is known as trans-to-cis isomerisation. We’re the first to succeed in taking real-time snapshots of this type of reaction.” This type of isomerisation is also what gives vision – in that case the retinal chromophore undergoes a cis-to-trans isomerisation that ultimately leads to neuronal excitation in the eye.
“We were able to obtain detailed structures at incredibly short time points after the initial absorption event by taking flash X-ray snapshots with the world’s brightest X-ray source,” said co-author Henry Chapman from CFEL at DESY. But, as Pande pointed out, “these are very challenging experiments, where we needed considerable innovation to assign the correct time stamps to hundreds of thousands of X-ray patterns.”
The researchers had already studied light-induced structural changes in PYP at LCLS before, revealing atomic motions as fast as 10 billionths of a second (10 nanoseconds). By tweaking their experiment with a faster optical laser and better timing tools and sorting, they were now able to improve their speed limit 100,000 times and capture reactions in the protein that are 1,000 times faster than any seen in an X-ray experiment before.
“The new data show for the first time how the bacterial sensor reacts immediately after it absorbs light,” says co-author Andy Aquila from SLAC. “The initial response, which is almost instantaneous, is absolutely crucial because it creates a ripple effect in the protein, setting the stage for its biological function.”
The technique could prove valuable to unveil a number of other important ultrafast light-driven processes, for instance how visual pigments in the human eye respond to light, and how absorbing too much of it damages them; how photosynthetic organisms turn light into chemical energy, a process that could serve as a model for the development of new energy technologies; or how atomic structures respond to light pulses of different shape and duration, an important first step toward controlling chemical reactions with light.