Putting antibiotic-resistant bacteria and the immune system under 'surveillance'
Multi-disciplinary team looks to develop new tools to forecast and treat antibiotic-resistant bacteria
A research team led by a Boston College biologist will use a $10-million National Institutes of Health grant to study the role of the immune system in the emergence of antibiotic-resistant bacteria.
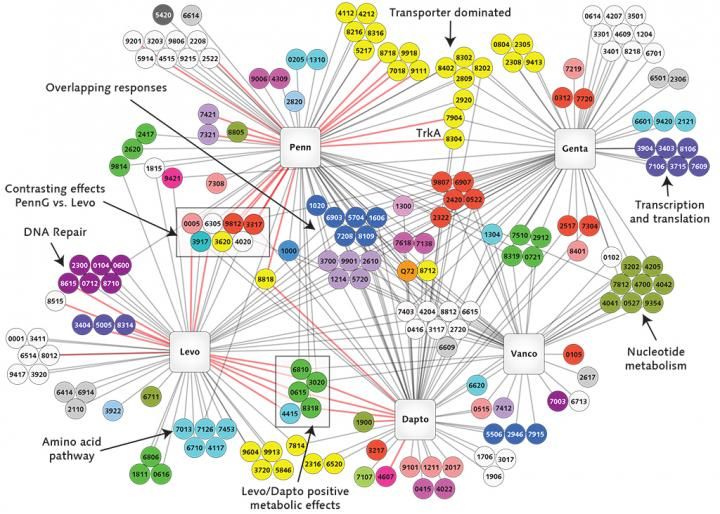
This illustration, developed through the research of Boston College Assistant Professor of Biology Tim van Opijnen, reflects the interactions of the genes of the potentially deadly bacterium Streptococcus pneumoniae with five antibiotics represented in the squares (clockwise from lower left: levofloxacin, penicillin, gentamicin, vancomycin, and daptomycin). The circles represent individual genes, colored to denote their functions -- such as yellow for transport of materials; pale lavender for cell division. A black line shows that a gene is involved in protecting the bacterium from the antibiotic; a red line means the gene makes the bacterium more susceptible to the antibiotic. If any one of the six dark blue genes at the center are absent or damaged, bacterial growth slows in the presence of the antibiotics. These particular genes are involved in forming a structural shell around the bacterial cell, and van Opijnen says their products (e.g., proteins) may present a prime target for antibiotic attack. The seven genes in the box at center-left exhibit mixed interactions depending on the antibiotic. Van Opijnen says this might have value in that it could be "extremely confusing" for the bacterium, repressing its evolution toward resistance to penicillin and levofloxacin. (courtesy Boston College Magazine)
Boston College
The research could serve as the foundation for the development of technological applications to help scientists forecast drug resistance in certain bacteria and identify effective courses of treatment for bacterial infections.
"There are three factors at play: host, bacteria and antibiotic," said Boston College's Tim van Opijnen, a microbial systems biologist who uses robotics, high-throughput sequencing and computational methods to study bacteria and antibiotic resistance. "In reality, we have very little understanding of how these three work in relation to and with each other."
Since their introduction 70 years ago, antibiotics have been used to treat patients with bacterial infections. Over time, many infectious organisms have adapted to the drugs designed to kill them, making those treatments less effective.
Van Opijnen, an assistant professor of biology and the principal investigator on the project will team up with BC computer scientist José Bento and researchers at Tufts Medical School, St. Jude Children's Hospital in Memphis and the Pittsburgh School of Medicine.
The project will use cutting-edge genomics research tools and techniques, including transposon sequencing, or Tn-seq, a technique that can quickly comb through millions of genetic sequences and single out gene functions in bacteria. Van Opijnen has developed Tn-seq, which he has used to identify certain genes in bacteria that foster their escape from the disease-fighting capabilities of the immune system.
"We want to comprehensively determine how bacteria interact with the immune system of the patient and how these interactions permit or prevent the evolution of antibiotic resistance," said van Opijnen.
The team will use high-volume genomics sequencing techniques, full-genome sequencing, immune system monitoring, neural networks and active learning techniques during the five-year study, which has been funded by the NIH's National Institute of Allergy and Infectious Diseases.
The grant brings together evolutionary biologists, infectious disease specialists, and computer scientists in an effort to improve the use of the massive quantities of data produced by high-throughput sequencing, van Opijnen said.
"Biology is flooded with data, but our analytical tools can be much more sophisticated than they are now," he said. "What we need to reach that level of sophistication is a cross-over to other disciplines that doesn't happen often enough now. This project is a chance to make those connections."
In addition to developing a computer model to guide optimal treatment, van Opijnen said additional treatment applications could include increased accuracy predicting resistance emergence and the development of new antibiotic targets and treatment strategies.
Central to reducing the toll of antibiotic resistance is understanding how patient, bacteria and drug interact.
"The immune system is essential in getting rid of infection. It is not just the drug," said van Opijnen. "These drugs lower the volume of bacteria in the body so the immune system can clear out the rest. But the immune system needs to cooperate with the drug to be successful."