Candidalysin – the first toxin of Candida albicans
Team of German and British scientists discovers tissue-damaging fungal toxin
In a pioneering study, scientists in Jena, Borstel, Aberdeen and London have discovered a toxin in the fungus Candida albicans, which plays a crucial role during human mucosal infection.
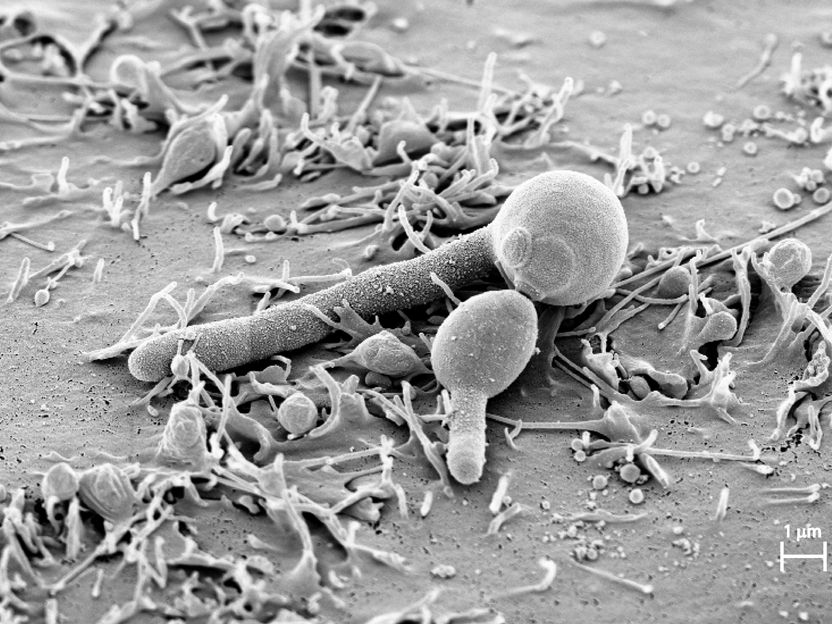
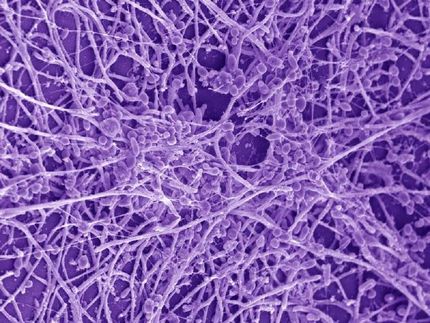
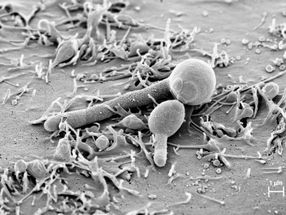
Candida albicans attached to the oral mucosa as a yeast cell and forms a filamentous hypha. The hypha produces the toxin Candidalysin, which has now been discovered by a team of German and British researchers.
Holland, Özel, Zakikhany, Hube
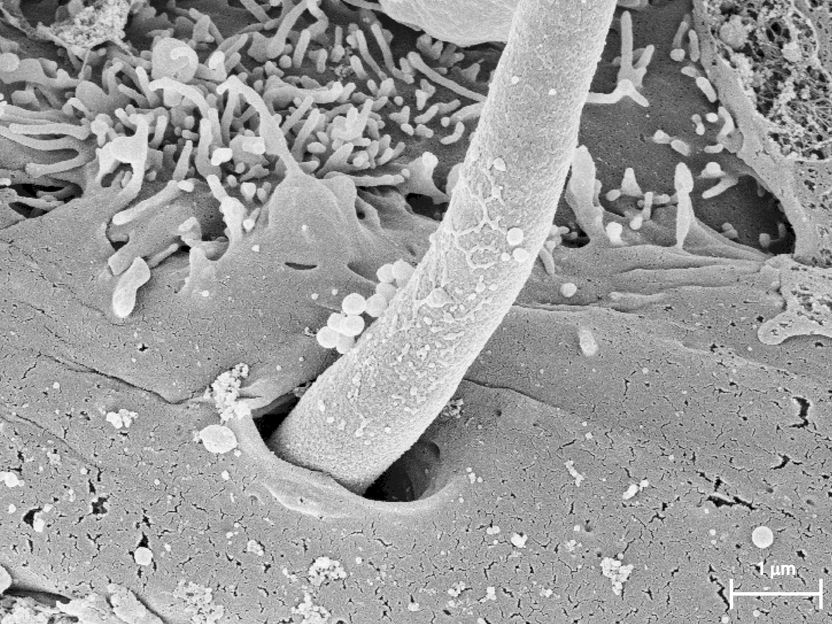
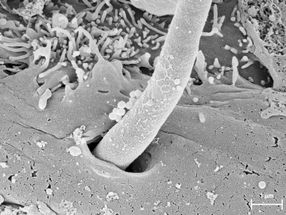
A filamentous hypha formed by Candida albicans enters a cell of the oral mucosa. During this invasion it produces the toxin Candidalysin, as shown by an international team of researchers.
Holland, Özel, Zakikhany und Hube
Throughout evolution, pathogens have come up with many tricks to infect and damage their hosts: viruses capture whole cells and turn them into factories for their own replication until the cells are exhausted and die. Infectious bacteria produce multiple molecules that can manipulate the host cell’s metabolism or simply destroy it. But what about human pathogenic fungi?
It is well known that certain fungi produce poisons which, if accidentally consumed, can result in sickness or even death. However, up until now, production of host cell-destroying toxins by the microscopic fungi that can infect us has never been shown. For decades, scientists have been looking for the molecules that are directly responsible for tissue damage during the course of fungal diseases. However, they did not find toxins in pathogenic fungi, which directly damages their infected hosts, and distinguishes these dangerous species from their harmless relatives. This is regrettable, since such knowledge would have been of great value for understanding human fungal infections and for taking therapeutic countermeasures.
Hence, the discovery by a German-British team of researchers came as quite a surprise. Microbiologists from Jena, Borstel, Aberdeen and London succeeded for the first time in identifying a real toxin in Candida albicans – a usually harmless gut-dwelling yeast which frequently causes diseases such as thrush. Candidalysin, as the new toxin was called, destroys human cells by forming holes in their membranes. The team of scientists elucidated this mechanism using cells of the oral mucosa as a model. Oral infections with Candida albicans commonly occur in HIV patients, but also in very young and elderly people with weakened immune systems.
The initial impulse for the discovery came from Julian Naglik’s research group at King’s CollegeLondon, who has been studying how human oral cells respond to fungal infections. The team of Bernhard Hube at the Leibniz Institute for Natural Product Research and Infection Biology – Hans Knöll Institute – (HKI) in Jena, Germany, investigated the interactions between fungus and host on a molecular level and demonstrated that Candidalysin actually damages the host cell. In addition, the biophysicist Thomas Gutsmann at the Leibniz-Center for Medicine and Biosciences in Borstel and his group studied the precise interaction between the fungal toxin and the cell membrane. Additional researchers in the UK and the USA also contributed data. The discovery of Candidalysin was made possible by a successful combination of individual expertise.
But why did it take researchers several decades of intensive investigations to find the crucial molecule? Similar toxic compounds – peptides – have long been known from other microbial pathogens. Here, the trick is that Candida albicans initially produces a much larger molecule – a polypeptide. The gene encoding for this polypeptide has long been known; however, its function remained elusive. Only now, using state-of-the-art analytics and building on recent insights, Naglik, Hube and their colleagues could successfully detect and characterize the small molecule. They realized that the polypeptide is cut into small pieces inside the fungus by a certain enzyme, but only one of these peptide fragments is the newly discovered toxin Candidalysin. The scientists chose this particular name because the peptide contributes to the destruction – the “lysis” – of the cell. From a harmless precursor, the dangerous toxin is only produced when it is actually required by the pathogen.
The production of this toxin is tightly linked to a morphological switch, which is crucial for disease. Candida albicans grows in two different forms: either as an egg-shaped yeast cell or as a filamentous hyphal form. In the human body, small numbers of yeast cells are harmless and our immune systems realizes that it does not face any (immediate) danger. However, when invasive filaments are formed, they release Candidalysin, and it is the activity of the toxin itself that is recognized, acting as a “red alert” signal. Therefore, the immune system has learned to recognize Candidalysin in order to discriminate between harmless yeasts and invasive, toxin-producing “dangerous” filaments. “This is an excellent example of co-evolution”, says Bernhard Hube, Professor at Friedrich Schiller University Jena and department head at HKI. “The pathogen generates a toxin to harm its host and the host quickly senses the toxin and initiates countermeasures”. The biological function of the toxin during normal growth, when the fungus lives harmlessly on the mucosa currently remains unknown.
“These ground-breaking results in fungal infection biology clearly show that in addition to copious amounts of tenacity, the most important factor for scientific success is an excellent power of observation”, states Hube. “Every small result and every observation constantly has to be re-evaluated and re-interpreted until the molecular reality becomes visible. The outstanding international collaboration within our team of researchers has been crucial for our success. For the scientists from Leibniz Institutes this is their own special contribution to this year’s Leibniz Anniversary.”
The fungus Candida albicans and its toxin Candidalysin will continue to keep scientists from Jena, Borstel and London asking questions for many years to come. How does the toxin interact with the immune system? Does the toxin also have activity against bacteria and does this have any effects on common habitats such as in the human gut? What role do other genetic components of the fungus play during infection?