Ensuring the integrity of our genetic material during reproduction
Molecular Biologists investigate the process of cutting and pasting DNA
The genetic information we receive from our parents in the form of chromosomes are mosaics assembled from the two copies of chromosomes each parent has. This reshuffling of chromosome pieces happens via a cut and paste mechanism. How such cuts – or breaks – in our genetic material are repaired is the research interest of Verena Jantsch and her group at the Max F. Perutz Laboratories of the University of Vienna and the Medical University of Vienna. Their findings give important insights into the processes that ensure the integrity of our genetic material, preventing genetic disease and cancer development.
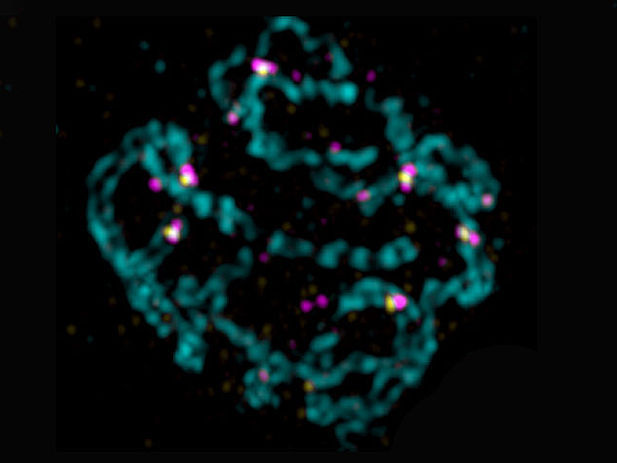
Picture of a nucleus, which will develop into an egg, imaged with structured Illumination Microscopy. It shows the aligned parental chromosomes (cyan). In yellow: the spots where DNA breaks will mature into a connection between the parental chromosomes. Surprisingly, this is where the RMI complex also acts (magenta).
Copyright: Alexander Woglar, MFPL/ Stanford University
Our genetic material – our DNA – must be stable; so that it can be passed on from generation to generation and life can persist. On the other hand, it must be versatile to allow for genetic variety and evolution. DNA breaks are introduced on purpose during reproduction to guarantee faithful chromosome distribution. But they also arise for example from damaging environmental factors or toxic metabolic products. Luckily, nature has devised a number of sophisticated processes that repair DNA breaks.
Mosaic DNA arises during the generation of egg and sperm cells
Since her Postdoc years, Verena Jantsch from the Max F. Perutz Laboratories (MFPL) has been interested in chromosomes and the processes ensuring their proper distribution into cells and their integrity. "DNA double strand breaks comprise one type of DNA damage. They are for example part of a cut and paste mechanism to create mosaic chromosomes and so introducing genetic variety in egg and sperm cells. In addition, they are crucial for connecting chromosomes – a precondition for accurate distribution of chromosomes into egg and sperm cells. They are repaired by a process called homologous recombination where the DNA damage is repaired from an identical copy of DNA present in the same cell," explains Verena Jantsch, one of the Berta Karlik Professors of the University of Vienna. Important for the outcome of homologous recombination is a machine called RTR complex, which consists of several protein factors. Mutations in these factors result in genetic instability and promote cancer development.
Keeping DNA breaks in check
Marlene Jagut, Postdoc in Verena Jantsch’s lab together with collaborators from Anne Villeneuve’s lab at Stanford University and Arndt von Haeseler at MFPL could now show that one of the RTR complex factors – RMI – has several crucial functions during the repair of DNA double strand breaks. She explains: "Using a genetic model system, we showed that RMI is required for defining the position and maturation of homologous recombination events along chromosomes. Mutations in RMI led to both undesirable connections between chromosomes and incompletely repaired DNA breaks, all leading to chromosomal abnormalities in the germ cells."
The findings not only contribute to our understanding of the role of the RTR complex in the generation of egg and sperm cells, but may also prove helpful in understanding cancer-related processes. In the future Verena Jantsch and her team would like to learn more about how the RTR complex directs the outcome of homologous recombination. Several advantages of their model system will allow them to study how the RTR complex contributes to the final steps during the maturation of the connection between chromosomes. This late function of the RTR machinery during recombination had gone unnoticed in previous studies.