A network for longevity
Transcription factors influence lifespan of roundworms
Gradual disruption of various processes in our cells results in ageing. Now scientists at the Max Planck Institute for Biology of Ageing in Cologne have discovered a network of regulatory molecules that converge to prevent this.
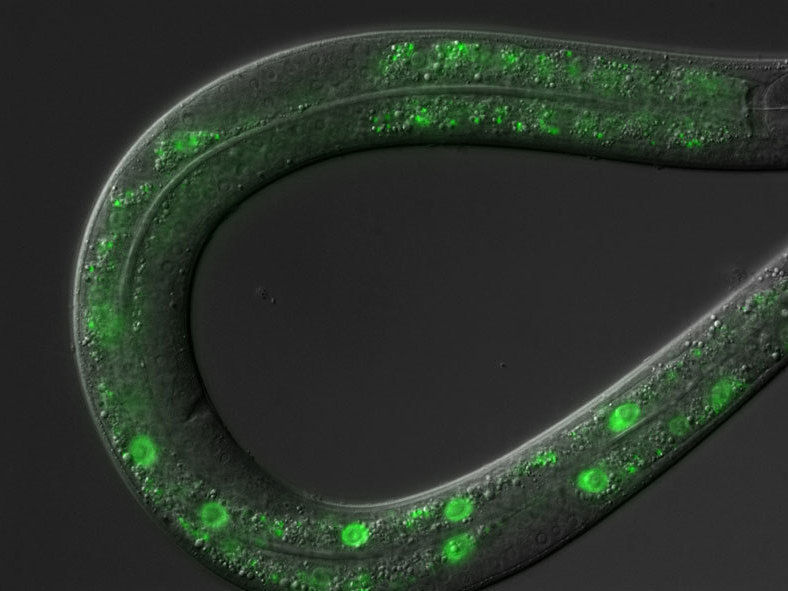
C. elegans roundworm: green fluorescence labels one of the transcription factors.
© MPI f. Biology of Ageing
An ageing cell goes through major negative changes: defective proteins are not eliminated as they should, mitochondria do not function properly, the ability to sense nutrients is lost. All of these defects lead to shortened lifespan. One might think at first glance, they appear to have nothing to do with each other on a molecular level. “In fact, they are highly interconnected”, says Adam Antebi, Director at the Max Planck Institute for Biology of Ageing in Cologne. “Now we have found a network of regulators that connects all those different cellular processes.”
For their studies the researchers used the roundworm Caenorhabditis elegans, a commonly used model organism in the field of ageing research. It all began with a scientific finding scientists made already some years ago: Roundworms live much longer if you remove their germ cells – the sperm and egg producing cells. “But we did not know why this happened”, explains Antebi. To answer this question the scientists removed specific genes to test if these worms lost the ability to live long. If this was the case, the researchers assumed that they found a gene that was normally required to increase the lifespan. In the end the researchers had a list of proteins, which extend lifetime. Many of them belonged to the so-called transcription factors - proteins that reside in the nucleus of the cell to turn on and off other genes.
The detected transcription factors appear to work together. “We found that all these transcription factors regulate and support one another. Actually they behave like a network”, Antebi says. This network impacts very diverse processes in the worm-cells: the recycling machine, the digestion system and the sensing of nutrients. “The end-result is changes in metabolism, the process where nutrients become the fuel and building blocks we need”, Antebi explains. With their study the researchers can begin to explain how reproduction, metabolism and life span are intertwined.
Original publication
Shuhei Nakamura, Özlem Karalay, Philipp S. Jäger, Makoto Horikawa, Corinna Klein, Kayo Nakamura, Christian Latza, Sven E. Templer, Christoph Dieterich, Adam Antebi; "Mondo complexes regulate TFEB via TOR inhibition to promote longevity in response to gonadal signals"; Nature Communications; 22 March 2016
Most read news
Original publication
Shuhei Nakamura, Özlem Karalay, Philipp S. Jäger, Makoto Horikawa, Corinna Klein, Kayo Nakamura, Christian Latza, Sven E. Templer, Christoph Dieterich, Adam Antebi; "Mondo complexes regulate TFEB via TOR inhibition to promote longevity in response to gonadal signals"; Nature Communications; 22 March 2016
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.