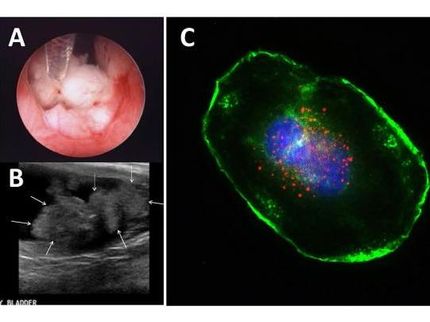
Clinical trial uses cold virus to attack bladder cancer
When people with stage 1 bladder cancer relapse after standard treatment, often the next step is to remove their bladders. Now a University of Florida Health researcher is testing whether a modified version of the common cold virus could prevent this step, or even cure the disease.
About 74,000 people in the United States are diagnosed with bladder cancer annually, according to the National Cancer Institute, and the cancer recurs at rates ranging from 30 to 80 percent, said Paul Crispen, M.D., a urologic oncologist with UF Health and an assistant professor in the UF College of Medicine’s department of urology. He is working with a company called Cold Genesys to open a clinical trial using a modified cold virus to kill cancer cells in the bladder.
The treatment being tested showed promise in fighting bladder cancer during a phase 1 study evaluating the safety of the treatment. While patients can relapse afterward, the method may offer better response rates than currently available medications, and researchers are studying ways to hone the treatment.
Physicians commonly treat patients with stage 1 bladder cancer by placing chemotherapy drugs within their bladder through a catheter. However, when the bladder cancer continues to recur following these treatments, physicians often recommend bladder removal. While bladder removal can cure patients with stage 1 bladder cancer, the surgery also results in dramatic changes in their quality of life.
“The most interesting aspect of this trial and the reason we have a lot of patients interested in participating is that this is a method that may allow a patient to keep their bladder in place,” Crispen said.
Now, Crispen and Cold Genesys hope that the modified cold virus could prove effective against bladder cancer and potentially decrease the chance of bladder removal. The virus is loaded directly into the bladder through a catheter, and is designed to replicate only within cancer cells, killing them.
The modified adenovirus is equipped with what’s called an S-1 promoter, which triggers the virus’ DNA to tell the virus to begin replicating within cancer cells. That causes those cells to burst open and release more viral particles, said Dominic Curran, M.B.Ch.B., the associate medical director for Cold Genesys.
The injury to the cells also prompts the cells to release cytokines, which are proteins involved in the immune response that help activate the immune system to further attack cancer cells.
“Because of the way it’s designed, the cold virus only affects mutating cells,” Curran said. “It’s designed to not infect normal, healthy cells.”
The initial phase 1 trial that examined the virus showed promise in a small group of people who were treated. Thirty-five patients received single or multiple treatments of the adenovirus at one of four dosage levels to assess whether they could tolerate the treatment well. In a follow-up assessment, 48 percent of these patients had a complete response, or no evidence of cancer in a follow-up appointment, Curran said. Of the 11 patients who were given multiple doses, nine had a complete response to the treatment. However, the median duration of the response was 10.4 months in both the single- and multiple-dose groups. As with other bladder treatments in which drugs are delivered through a catheter, side effects were noted, including bladder irritation and fatigue.
Curran said the modified cold virus could be used in approaches for other cancers.
“It’s quite easy to catch the common cold, and we would like to harness that and adjust the virus to infect and replicate within the tumor cells of different types of cancer,” Curran said.
Most read news
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Loving the sweet enemy - Foods rich in fats and carbohydrates stimulate the reward system in the brain particularly strong

First-in-class immunotherapies for the treatment of cancer and autoimmune diseases - ImmunOs Therapeutics Raises $74 Million Series B Financing Round
BioMed X and Boehringer Ingelheim start new joint research group
Nobilon advances first vaccine into human trials - Intranasal influenza vaccine begins Phase I clinical development
Raptor Pharmaceuticals and TorreyPines Therapeutics Receive Stockholder Approvals to Merge - Merger to Create NASDAQ-Listed Biopharmaceutical Company named Raptor Pharmaceutical Corp.
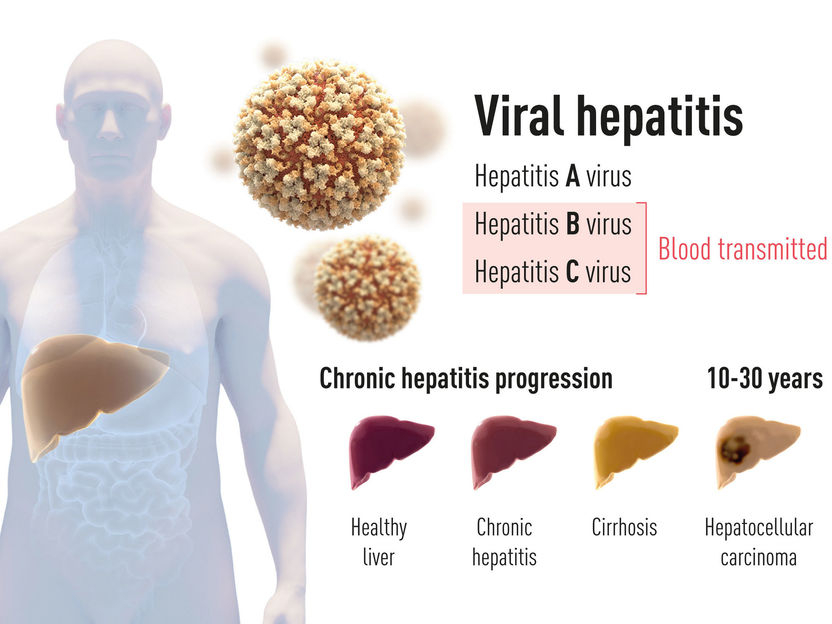
Nobel Prize for Physiology or Medicine 2020 Announced - Nobel Prize awarded to Harvey J. Alter, Michael Houghton and Charles M. Rice for the discovery of Hepatitis C virus
PharmAthene and SIGA Technologies sign definitive merger agreement
PerkinElmer announces third quarter results - GAAP Revenue of $548 million versus $563 million in the comparable prior period

Glox Therapeutics Secures £4.3M Seed Funding to Develop Precision Antimicrobials Targeting Drug-resistant Bacteria - Spin-out from the Universities of Glasgow and Oxford

Doped by food - Dopamine release regulates our eating behaviour

Turning fallen leaves into sustainably made paper - Ukrainian scientist selected as a finalist for the Young Inventors Prize 2024