Improving modern vaccines - sugar polymer tails wag the protein dog
Advertisement
Millions of people - particularly infants in underdeveloped countries -- suffer from the serious life threatening illnesses of meningitis, pneumonia and influenza. These are due to infection by microbes such as N. meninigitidis, S. pneumoniae and H. influenzae b.
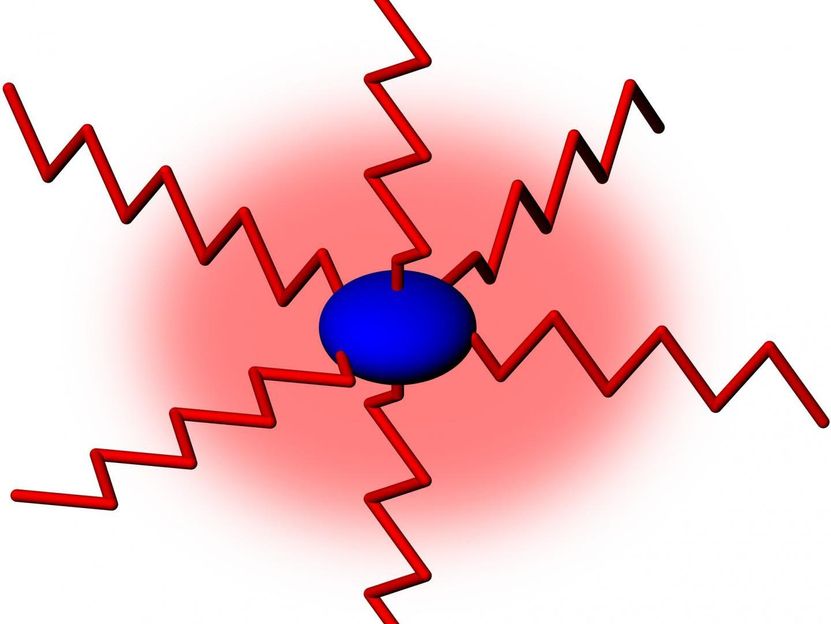
Caption to Figure: Schematic diagram of a Glycovaccine construct of sugar-polymer tails (shown as red zig-zag lines) connected to a globular protein core (shown in blue). The flexible sugar (carbohydrate) polymers control the physical properties in vaccine formulations and provide a protective cloud (red shade) against protein denaturation and vaccine effectiveness.
The University of Nottingham
Early vaccines were based on the large and complex carbohydrate (sugar) based polymers produced by the bacteria. More recently new glycoconjugate vaccines have been developed which involves 'fusing' the complex carbohydrates -- the sugar polymer tails -- onto carrier proteins. These sugar-protein complexes improve the effectiveness and longevity of the vaccine. However, there are still problems concerning the stability of formulations they are prepared in.
Scientists at The University of Nottingham's National Centre for Macromolecular Hydrodynamics have just published the third in a series of papers showing that the sugar chains control the physical behaviour of these vaccines in the aqueous preparations used in their delivery. It is hoped this research into the hydrodynamic properties of vaccine preparations will help lead to the development of improved and more stable vaccines.
The latest study led by Steve Harding, Professor of Applied Biochemistry in the School of Biosciences and Dr Gary Adams an expert in Diabetes Health and Therapeutics in the School of Health Sciences, was carried out in collaboration with colleagues at GSK vaccines in Belgium.
Using the latest 'hydrodynamic' techniques and approaches -- many of which have been developed in their laboratory -- Professor Harding and Dr Adams found that the protein has a compact globular conformation whereas the carbohydrate polymers have much more extended flexible structures.
The conjugates were not only found to be over 50 times larger than the protein (and about 10 times larger than the individual carbohydrate polymers) but also retained the highly flexible properties of the carbohydrate: the attached carbohydrate chains flop all over the place effectively encapsulating the protein and protecting it from denaturation, which can cause breakage and loss of effectiveness. Conjugation strategies need to ensure the carbohydrate chains are long enough to achieve this coverage.
Professor Harding said: "We believe this is an exciting development. Carbohydrates are often underestimated in terms of their therapeutic importance -- they still have a sort of 'Cinderella' status in biochemistry.
"The stability of vaccine preparation is of particular concern under hot climate conditions and until now, nobody has been quite sure about the relative roles the sugar and protein components play in the properties of these substances. Understanding that would be a big step in producing more stable and effective vaccines."
Original publication
Ali Saber Abdelhameed, Gary G. Adams, Gordon A. Morris, Fahad M. Almutairi, Pierre Duvivier, Karel Conrath & Stephen E. Harding; "A glycoconjugate of Haemophilus influenzae Type b capsular polysaccharide with tetanus toxoid protein: hydrodynamic properties mainly influenced by the carbohydrate"; Scientific Reports; 2016